535125
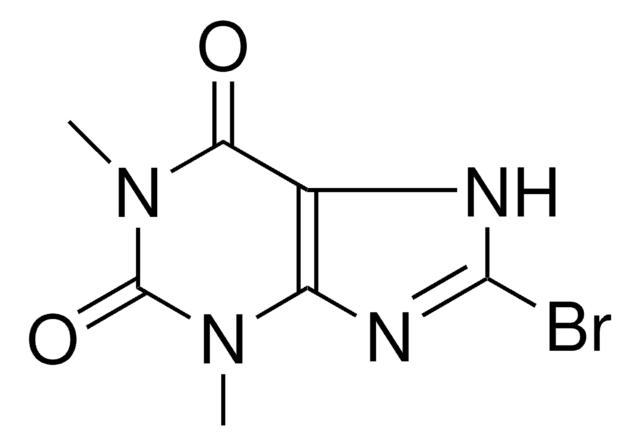
2-Bromohypoxanthine
96%
Se connecterpour consulter vos tarifs contractuels et ceux de votre entreprise/organisme
About This Item
Formule empirique (notation de Hill) :
C5H3BrN4O
Numéro CAS:
Poids moléculaire :
215.01
Numéro MDL:
Code UNSPSC :
12352100
ID de substance PubChem :
Nomenclature NACRES :
NA.22
Produits recommandés
Essai
96%
Pf
>350 °C (lit.)
Groupe fonctionnel
bromo
Chaîne SMILES
BrC1=Nc2nc[nH]c2C(=O)N1
InChI
1S/C5H3BrN4O/c6-5-9-3-2(4(11)10-5)7-1-8-3/h1H,(H2,7,8,9,10,11)
Clé InChI
ONXCBJOMYNPZNI-UHFFFAOYSA-N
Catégories apparentées
Mention d'avertissement
Warning
Mentions de danger
Conseils de prudence
Classification des risques
Eye Irrit. 2 - Skin Irrit. 2 - STOT SE 3
Organes cibles
Respiratory system
Code de la classe de stockage
11 - Combustible Solids
Classe de danger pour l'eau (WGK)
WGK 3
Point d'éclair (°F)
Not applicable
Point d'éclair (°C)
Not applicable
Équipement de protection individuelle
dust mask type N95 (US), Eyeshields, Gloves
Faites votre choix parmi les versions les plus récentes :
Déjà en possession de ce produit ?
Retrouvez la documentation relative aux produits que vous avez récemment achetés dans la Bibliothèque de documents.
R S Sodum et al.
Chemical research in toxicology, 11(12), 1453-1459 (1998-12-22)
2-Nitropropane, an industrial chemical and a hepatocarcinogen in rats, induces aryl sulfotransferase-mediated liver DNA and RNA base modifications [Sodum, R. S., Sohn, O. S., Nie, G., and Fiala, E. S. (1994) Chem. Res. Toxicol. 7, 344-351]. Two of these modifications
H Xu et al.
Journal of medicinal chemistry, 38(1), 49-57 (1995-01-06)
Two series of selective inhibitors of herpes simplex virus types 1 and 2 (HSV1,2) thymidine kinases (TK) have been developed as potential treatment of recurrent virus infections. Among compounds related to the potent base analog N2-[m-(trifluoromethyl)phenyl]guanine (mCF3-PG), none was a
Sheng Ding et al.
Journal of combinatorial chemistry, 4(2), 183-186 (2002-03-12)
A resin-capture and release strategy for making combinatorial 2,6,9-trisubstituted purine libraries is demonstrated by capturing N9-derivatized purines at the C6 position with a thio-modified polymer. The C2 fluoro group is subsequently substituted with primary and secondary amines followed by thioether
M M Butler et al.
Nucleic acids research, 18(24), 7381-7387 (1990-12-25)
6-(p-Hydroxyphenylhydrazino)uracil (H2-HPUra) is a selective and potent inhibitor of the replication-specific class III DNA polymerase (pol III) of Gr+ bacteria. Although formally a pyrimidine, H2-HPUra derives its inhibitory activity from its specific capacity to mimic the purine nucleotide, dGTP. We
Andrzej Manikowski et al.
Journal of medicinal chemistry, 48(11), 3919-3929 (2005-05-27)
Derivatives of the herpes simplex thymidine kinase inhibitor HBPG [2-phenylamino-9-(4-hydroxybutyl)-6-oxopurine] have been synthesized and tested for inhibitory activity against recombinant enzymes (TK) from herpes simplex types 1 and 2 (HSV-1, HSV-2). The compounds inhibited phosphorylation of [3H]thymidine by both enzymes
Notre équipe de scientifiques dispose d'une expérience dans tous les secteurs de la recherche, notamment en sciences de la vie, science des matériaux, synthèse chimique, chromatographie, analyse et dans de nombreux autres domaines..
Contacter notre Service technique