393274
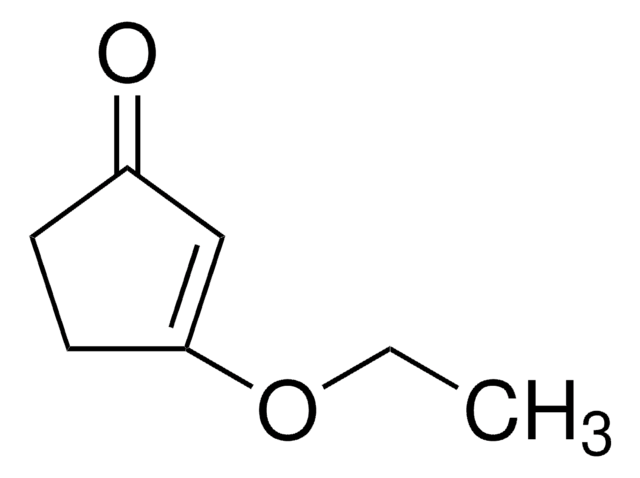
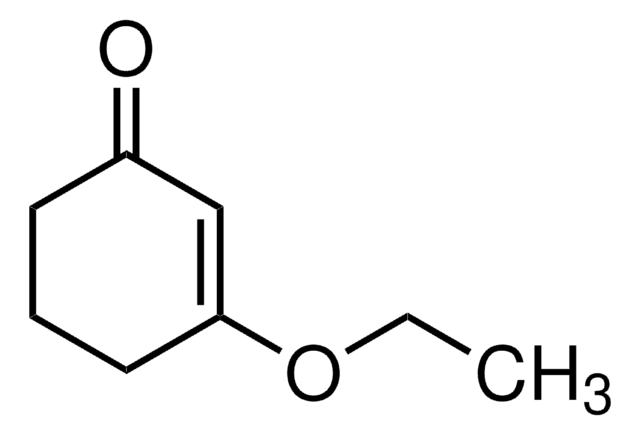
3-Methoxy-2-cyclopenten-1-one
99%
Se connecterpour consulter vos tarifs contractuels et ceux de votre entreprise/organisme
About This Item
Formule linéaire :
CH3OC5H5(=O)
Numéro CAS:
Poids moléculaire :
112.13
Numéro MDL:
Code UNSPSC :
12352100
ID de substance PubChem :
Nomenclature NACRES :
NA.22
Produits recommandés
Niveau de qualité
Essai
99%
Forme
solid
Pf
49-53 °C (lit.)
Groupe fonctionnel
ether
ketone
Chaîne SMILES
COC1=CC(=O)CC1
InChI
1S/C6H8O2/c1-8-6-3-2-5(7)4-6/h4H,2-3H2,1H3
Clé InChI
DTWCFCILAJVNPE-UHFFFAOYSA-N
Catégories apparentées
Description générale
3-Methoxy-2-cyclopenten-1-one (3-methoxycyclopent-2-enone) is a 3-methoxycycloalk-2- enone.
Application
3-Methoxy-2-cyclopenten-1-one (3-methoxycyclopent-2-enone) may be used in the following studies:
- Synthesis of 3-cyclopentyl-2-cyclopenten-1-one.
- As a starting material in the synthesis of 3-alkyl-2-aryl-2-cyclopenten-1-one oxime derivatives and kjellmanianone, an antibiotic.
- As one of the reagent in the synthesis of 3-aryl enones.
- Preparation of 1:1 diastereomeric mixture of TBS (tert-butyldimethylsilyl)-protected enones.
Code de la classe de stockage
11 - Combustible Solids
Classe de danger pour l'eau (WGK)
WGK 3
Point d'éclair (°F)
Not applicable
Point d'éclair (°C)
Not applicable
Équipement de protection individuelle
Eyeshields, Gloves, type N95 (US)
Faites votre choix parmi les versions les plus récentes :
Déjà en possession de ce produit ?
Retrouvez la documentation relative aux produits que vous avez récemment achetés dans la Bibliothèque de documents.
Les clients ont également consulté
Patrick Y Toullec et al.
Proceedings of the National Academy of Sciences of the United States of America, 101(16), 5810-5814 (2004-04-09)
The enantioselective formation of a quaternary stereogenic center coinciding with a hydroxylation process is a very rare reaction from a homogeneous catalysis point of view. Indeed, to our knowledge, no asymmetric transition-metal-catalyzed direct hydroxylation has been reported before. We describe
Tetrahedron, 47, 173-173 (1991)
Discovery of orally efficacious melanin-concentrating hormone receptor-1 antagonists as antiobesity agents. Synthesis, SAR, and biological evaluation of bicyclo [3.1. 0] hexyl ureas.
McBriar MD, et al.
Journal of Medicinal Chemistry, 49(7), 1202-1207 (2012)
Yeonjoon Kim et al.
Bioorganic & medicinal chemistry letters, 24(13), 2807-2810 (2014-05-24)
3-Alkyl-2-aryl-2-cyclopenten-1-one oxime derivatives (1) were studied as a novel class of inhibitors of tumor necrosis factor α (TNF-α) with regard to synthesis and in vitro SAR inhibition of TNF-α. The in vitro IC50 values of these compounds in rat and
Gamal A I Moustafa et al.
The Journal of organic chemistry, 77(2), 1202-1207 (2012-01-03)
The alkylation of dienolates generated from 3-methoxycycloalk-2-enones having a 3'-hydroxyl alkenyl chain provides the corresponding quaternized cycloalkenones in a highly diastereoselective manner. The high degree of stereocontrol in the α-quaternization possibly implies intervention of a rigid chelating transition state that
Notre équipe de scientifiques dispose d'une expérience dans tous les secteurs de la recherche, notamment en sciences de la vie, science des matériaux, synthèse chimique, chromatographie, analyse et dans de nombreux autres domaines..
Contacter notre Service technique