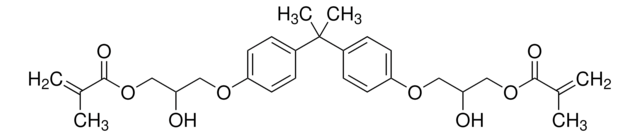
234907
2-(Dimethylamino)ethyl methacrylate
contains 700-1000 ppm monomethyl ether hydroquinone as inhibitor, 98%
Synonyme(s) :
Methacrylic acid 2-(dimethylamino)ethyl ester
About This Item
Produits recommandés
Densité de vapeur
5.4 (vs air)
Niveau de qualité
Pression de vapeur
<1 mmHg ( 25 °C)
Pureté
98%
Forme
liquid
Contient
700-1000 ppm monomethyl ether hydroquinone as inhibitor
Indice de réfraction
n20/D 1.439 (lit.)
Point d'ébullition
182-192 °C (lit.)
Densité
0.933 g/mL at 25 °C (lit.)
Température de stockage
2-8°C
Chaîne SMILES
CN(C)CCOC(=O)C(C)=C
InChI
1S/C8H15NO2/c1-7(2)8(10)11-6-5-9(3)4/h1,5-6H2,2-4H3
Clé InChI
JKNCOURZONDCGV-UHFFFAOYSA-N
Vous recherchez des produits similaires ? Visite Guide de comparaison des produits
Description générale
Application
- Quaternized poly (DMAEMA) can be used to prepare highly efficient antibacterial magnetic particles. The high density of quaternary ammonium groups generated via surface-initiated ATRP are responsible for high antibacterial activity.
- Ag nanoparticles immobilized into a poly (DMAEMA) brush layer can be used as a sensor platform for the detection of organic molecules by surface-enhanced Raman spectroscopy (SERS).
- It can also be used to prepare stable polymer-based gene delivery systems.
Mention d'avertissement
Danger
Mentions de danger
Conseils de prudence
Classification des risques
Acute Tox. 4 Dermal - Acute Tox. 4 Oral - Eye Dam. 1 - Skin Corr. 1B - Skin Sens. 1
Code de la classe de stockage
6.1A - Combustible acute toxic Cat. 1 and 2 / very toxic hazardous materials
Classe de danger pour l'eau (WGK)
WGK 1
Point d'éclair (°F)
147.2 °F - closed cup
Point d'éclair (°C)
64 °C - closed cup
Équipement de protection individuelle
Eyeshields, Faceshields, Gloves, type ABEK (EN14387) respirator filter
Faites votre choix parmi les versions les plus récentes :
Déjà en possession de ce produit ?
Retrouvez la documentation relative aux produits que vous avez récemment achetés dans la Bibliothèque de documents.
Les clients ont également consulté
Articles
The Progress in Development of Dental Restorative Materials
Notre équipe de scientifiques dispose d'une expérience dans tous les secteurs de la recherche, notamment en sciences de la vie, science des matériaux, synthèse chimique, chromatographie, analyse et dans de nombreux autres domaines..
Contacter notre Service technique![N-[3-(Dimethylamino)propyl]methacrylamide 99%, contains MEHQ as inhibitor](/deepweb/assets/sigmaaldrich/product/structures/295/145/6b4aae15-7cb5-4b7b-9c06-8e6d24e50951/640/6b4aae15-7cb5-4b7b-9c06-8e6d24e50951.png)
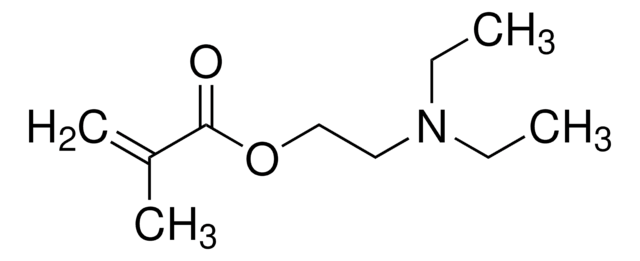
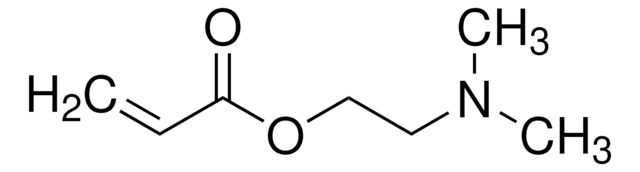
![[2-(Methacryloyloxy)ethyl]trimethylammonium chloride solution 75 wt. % in H2O](/deepweb/assets/sigmaaldrich/product/structures/316/612/66b0f4cf-d060-427d-b4f5-e8fab3e5cffe/640/66b0f4cf-d060-427d-b4f5-e8fab3e5cffe.png)
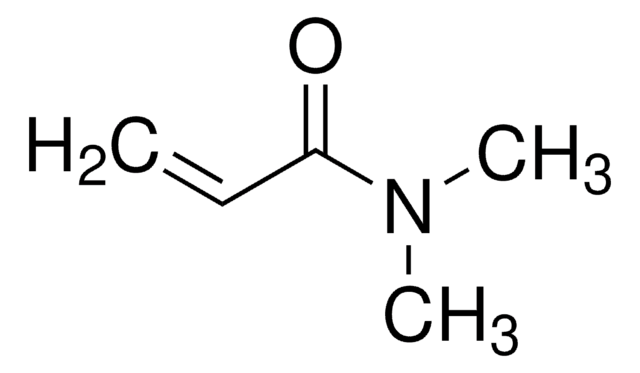





![[2-(Methacryloyloxy)ethyl]dimethyl-(3-sulfopropyl)ammonium hydroxide 95%](/deepweb/assets/sigmaaldrich/product/structures/217/219/73c91e1c-0ee4-4b3d-bead-a6dc3d09d1da/640/73c91e1c-0ee4-4b3d-bead-a6dc3d09d1da.png)