222410
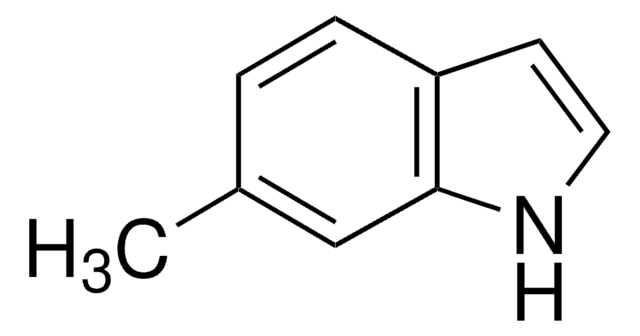
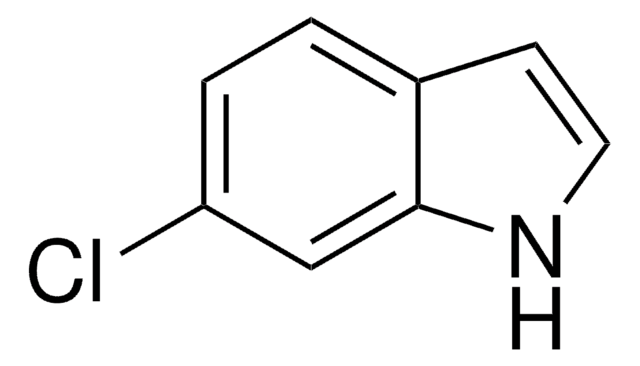
5-Methylindole
99%
Synonyme(s) :
NSC 522562
Se connecterpour consulter vos tarifs contractuels et ceux de votre entreprise/organisme
About This Item
Formule empirique (notation de Hill):
C9H9N
Numéro CAS:
Poids moléculaire :
131.17
Numéro CE :
Numéro MDL:
Code UNSPSC :
12352100
ID de substance PubChem :
Nomenclature NACRES :
NA.22
Produits recommandés
Niveau de qualité
Essai
99%
Forme
solid
Pf
60-62 °C (lit.)
Chaîne SMILES
Cc1ccc2[nH]ccc2c1
InChI
1S/C9H9N/c1-7-2-3-9-8(6-7)4-5-10-9/h2-6,10H,1H3
Clé InChI
YPKBCLZFIYBSHK-UHFFFAOYSA-N
Description générale
The binding of 5-methylindole (inducer) to the Escherichia coli trp repressor has been studied. The mass analyzed threshold ionization spectra of jetcooled 5-methylindole (5MI) has also been studied.
Application
Reactant for preparation of:
- Pharmaceutically active 2-oxo-1-pyrrolidine analogues
- Potential anticancer immunomodulators
- Preparation of antifungal agents
- Sodium-dependent glucose co-transporter 2 (SGLT2) inhibitors for the management of hyperglycemia in diabetes
- IL2-inducible T-cell kinase (ITK) inhibitors
- Checkpoint 1 kinase inhibitors
- CRTh2 antagonists
- Inhibitors of human immunodeficiency virus type 1 (HIV-1) attachment
- Agonists of the histamine H4 receptor
- Monoamine reuptake inhibitors
Mention d'avertissement
Warning
Mentions de danger
Conseils de prudence
Classification des risques
Eye Irrit. 2 - Skin Irrit. 2 - STOT SE 3
Organes cibles
Respiratory system
Code de la classe de stockage
11 - Combustible Solids
Classe de danger pour l'eau (WGK)
WGK 3
Équipement de protection individuelle
dust mask type N95 (US), Eyeshields, Gloves
Faites votre choix parmi les versions les plus récentes :
Déjà en possession de ce produit ?
Retrouvez la documentation relative aux produits que vous avez récemment achetés dans la Bibliothèque de documents.
Les clients ont également consulté
Changjiang Dong et al.
Science (New York, N.Y.), 309(5744), 2216-2219 (2005-10-01)
Chlorinated natural products include vancomycin and cryptophycin A. Their biosynthesis involves regioselective chlorination by flavin-dependent halogenases. We report the structural characterization of tryptophan 7-halogenase (PrnA), which regioselectively chlorinates tryptophan. Tryptophan and flavin adenine dinucleotide (FAD) are separated by a 10
Jung Lee Lin et al.
The Journal of chemical physics, 120(11), 5057-5063 (2004-07-23)
The vibrationally resolved mass analyzed threshold ionization spectra of jetcooled 5-methylindole (5MI) and 3-methylindole (3MI) have been recorded by ionizing via various vibronic levels of each species. The adiabatic ionization energies (IEs) of 5MI and 3MI are determined to be
P Babitzke et al.
The Journal of biological chemistry, 270(21), 12452-12456 (1995-05-26)
A filter binding assay was used to determine the structural features of L-tryptophan required for activation of TRAP, the trp RNA-binding attenuation protein of Bacillus subtilis. We examined the ability of L-tryptophan and 26 of its analogs to activate TRAP.
F Peter Guengerich et al.
Journal of medicinal chemistry, 47(12), 3236-3241 (2004-05-28)
Indigoids, a class of bis-indoles, represent a promising protein kinase inhibitor scaffold. Oxidation of indole by cytochrome P450 (P450) has been shown to generate species (indoxyl, isatin) that couple to yield indigo and indirubin. Escherichia coli-expressed human P450 2A6 mutants
Dorleta Gonzalez et al.
Bioorganic & medicinal chemistry, 26(9), 2551-2560 (2018-04-17)
Following the premises of the multitarget-directed ligands approach for the drug R&D against neurodegenerative diseases, where Alzheimer's disease (AD) outstands, we have synthesized and evaluated analogues of the gramine derivative ITH12657 (1-benzyl-5-methyl-3-(piperidin-1-ylmethyl-1H-indole, 2), which had shown important neuroprotective properties, such
Notre équipe de scientifiques dispose d'une expérience dans tous les secteurs de la recherche, notamment en sciences de la vie, science des matériaux, synthèse chimique, chromatographie, analyse et dans de nombreux autres domaines..
Contacter notre Service technique