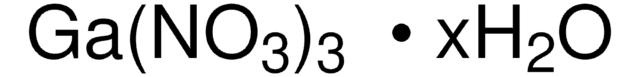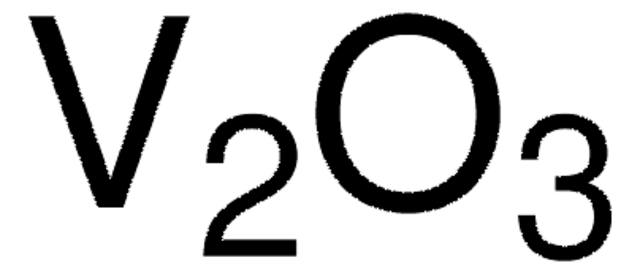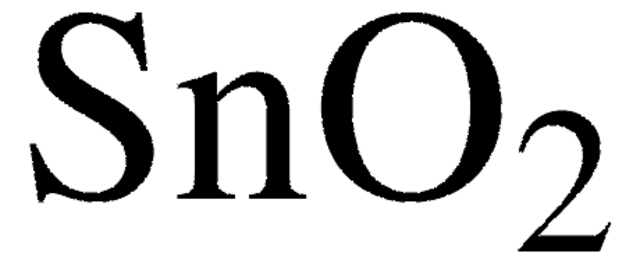215066
Gallium(III) oxide
≥99.99% trace metals basis
Synonyme(s) :
Gallium trioxide
About This Item
Produits recommandés
Niveau de qualité
Pureté
≥99.99% trace metals basis
Forme
(crystalline powder)
Pertinence de la réaction
reagent type: catalyst
core: gallium
Densité
5.88 g/mL at 25 °C
Chaîne SMILES
O=[Ga]O[Ga]=O
InChI
1S/2Ga.3O
Clé InChI
QZQVBEXLDFYHSR-UHFFFAOYSA-N
Vous recherchez des produits similaires ? Visite Guide de comparaison des produits
Catégories apparentées
Description générale
Application
Due to its distinct optical and electrical properties like moderate conductivity and high laser damage threshold, Ga2O3 can be used in laser-driven electron accelerators, low-loss plasmonics, and Si-based dielectric laser accelerators.
It can also be used as an effective catalyst for the dehydrogenation of propane to propene.
Code de la classe de stockage
11 - Combustible Solids
Classe de danger pour l'eau (WGK)
WGK 2
Point d'éclair (°F)
Not applicable
Point d'éclair (°C)
Not applicable
Équipement de protection individuelle
Eyeshields, Gloves, type N95 (US)
Certificats d'analyse (COA)
Recherchez un Certificats d'analyse (COA) en saisissant le numéro de lot du produit. Les numéros de lot figurent sur l'étiquette du produit après les mots "Lot" ou "Batch".
Déjà en possession de ce produit ?
Retrouvez la documentation relative aux produits que vous avez récemment achetés dans la Bibliothèque de documents.
Les clients ont également consulté
Notre équipe de scientifiques dispose d'une expérience dans tous les secteurs de la recherche, notamment en sciences de la vie, science des matériaux, synthèse chimique, chromatographie, analyse et dans de nombreux autres domaines..
Contacter notre Service technique