108723
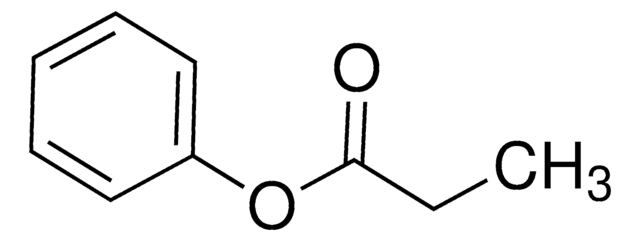
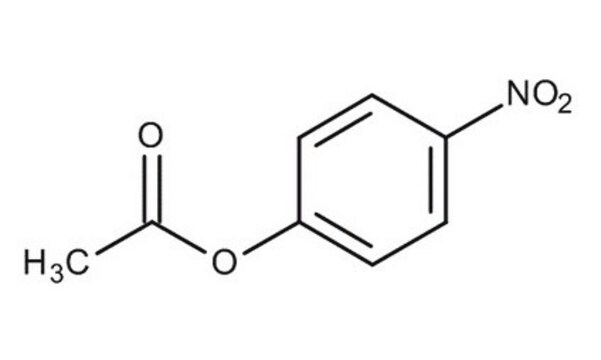
Phenyl acetate
99%
Synonyme(s) :
Acetic acid phenyl ester
About This Item
Produits recommandés
Niveau de qualité
Pureté
99%
Indice de réfraction
n20/D 1.501 (lit.)
Point d'ébullition
196 °C (lit.)
Densité
1.073 g/mL at 25 °C (lit.)
Groupe fonctionnel
ester
phenoxy
Chaîne SMILES
CC(=O)Oc1ccccc1
InChI
1S/C8H8O2/c1-7(9)10-8-5-3-2-4-6-8/h2-6H,1H3
Clé InChI
IPBVNPXQWQGGJP-UHFFFAOYSA-N
Informations sur le gène
human ... PON1(5444)
Vous recherchez des produits similaires ? Visite Guide de comparaison des produits
Catégories apparentées
Description générale
Mention d'avertissement
Warning
Mentions de danger
Classification des risques
Acute Tox. 4 Oral
Code de la classe de stockage
10 - Combustible liquids
Classe de danger pour l'eau (WGK)
WGK 2
Point d'éclair (°F)
170.6 °F - closed cup
Point d'éclair (°C)
77 °C - closed cup
Équipement de protection individuelle
Eyeshields, Faceshields, Gloves, type ABEK (EN14387) respirator filter
Faites votre choix parmi les versions les plus récentes :
Déjà en possession de ce produit ?
Retrouvez la documentation relative aux produits que vous avez récemment achetés dans la Bibliothèque de documents.
Les clients ont également consulté
Articles
The Fries rearrangement reaction is an organic name reaction which involves the conversion of phenolic esters into hydroxyaryl ketones on heating in the presence of a catalyst. Suitable catalysts for this reaction are Brønsted or Lewis acids such as HF, AlCl3, BF3, TiCl4, or SnCl4. The Fries rearrangement reaction is an ortho, para-selective reaction, and is used in the preparation of acyl phenols. This organic reaction has been named after German chemist Karl Theophil Fries.
The Fries rearrangement reaction is an organic name reaction which involves the conversion of phenolic esters into hydroxyaryl ketones on heating in the presence of a catalyst. Suitable catalysts for this reaction are Brønsted or Lewis acids such as HF, AlCl3, BF3, TiCl4, or SnCl4. The Fries rearrangement reaction is an ortho, para-selective reaction, and is used in the preparation of acyl phenols. This organic reaction has been named after German chemist Karl Theophil Fries.
Notre équipe de scientifiques dispose d'une expérience dans tous les secteurs de la recherche, notamment en sciences de la vie, science des matériaux, synthèse chimique, chromatographie, analyse et dans de nombreux autres domaines..
Contacter notre Service technique