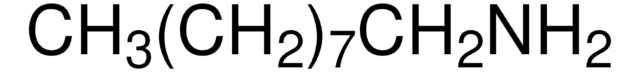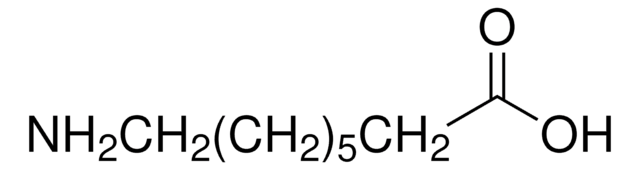About This Item
Recommended Products
Quality Level
Assay
98%
form
liquid
refractive index
n20/D 1.433 (lit.)
bp
201 °C (lit.)
density
0.782 g/mL at 25 °C (lit.)
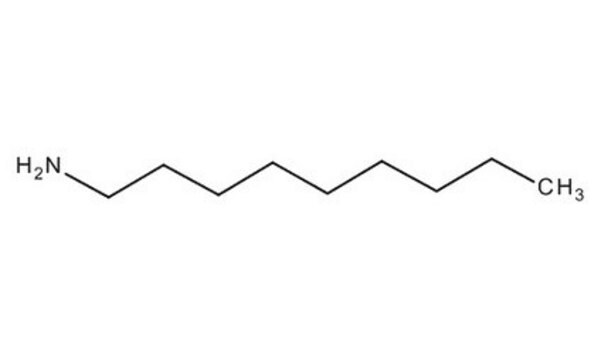
SMILES string
CCCCCCCCCN
InChI
1S/C9H21N/c1-2-3-4-5-6-7-8-9-10/h2-10H2,1H3
InChI key
FJDUDHYHRVPMJZ-UHFFFAOYSA-N
Looking for similar products? Visit Product Comparison Guide
Related Categories
General description
Application
- Contribution of liquid-phase and gas-phase ionization in extractive electrospray ionization mass spectrometry of primary amines.: This study discusses the dual ionization mechanisms in mass spectrometry, which could be crucial for analyzing chemical reactions involving nonylamine in both phases, providing a detailed methodology for chemists focused on reaction mechanisms and molecular analysis (Meier et al., 2011).
- Solvent-free derivatization of pristine multi-walled carbon nanotubes with amines.: This research provides insights into the solvent-free functionalization of carbon nanotubes with amines, including potentially nonylamine, which is significant for chemical engineers working on advanced material coatings and functionalities (Basiuk et al., 2005).
- Enhanced in vitro percutaneous penetration of salicylate by ion pair formation with alkylamines.: This article explores how alkylamines, possibly including nonylamine, enhance the penetration of salicylate through the skin, which is valuable for pharmaceutical scientists developing topical treatments (Kadono et al., 1998).
Signal Word
Danger
Hazard Statements
Precautionary Statements
Hazard Classifications
Acute Tox. 4 Dermal - Acute Tox. 4 Inhalation - Acute Tox. 4 Oral - Aquatic Acute 1 - Skin Corr. 1B
Storage Class Code
8A - Combustible corrosive hazardous materials
WGK
WGK 3
Flash Point(F)
165.2 °F - closed cup
Flash Point(C)
74 °C - closed cup
Personal Protective Equipment
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Protocols
Separation of Propylamine; Butylamine; Pentylamine; Hexylamine; Heptylamine; Octylamine; Nonylamine; Decylamine
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service