All Photos(1)
About This Item
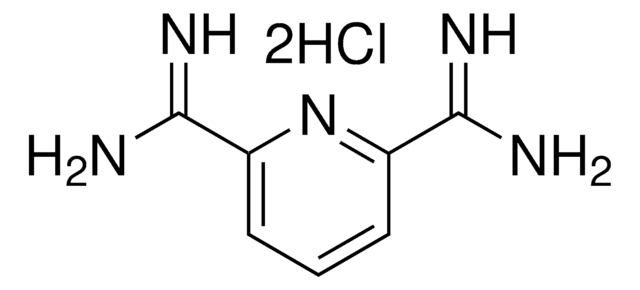
Empirical Formula (Hill Notation):
C7H7N3O2
CAS Number:
Molecular Weight:
165.15
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Assay
97%
mp
317 °C (dec.) (lit.)
functional group
amide
SMILES string
NC(=O)c1cccc(n1)C(N)=O
InChI
1S/C7H7N3O2/c8-6(11)4-2-1-3-5(10-4)7(9)12/h1-3H,(H2,8,11)(H2,9,12)
InChI key
UUVCRNTVNKTNRK-UHFFFAOYSA-N
General description
2,6-Pyridinedicarboxamide is an excellent tridentate ligand for transition metals (Cu2+, Ni2+).
Application
2,6-Pyridinedicarboxamide may be used in the preparation of new molecular turnstiles based on Sn-porphyrin derivatives.
Signal Word
Warning
Hazard Statements
Precautionary Statements
Hazard Classifications
Eye Irrit. 2 - Skin Irrit. 2 - STOT SE 3
Target Organs
Respiratory system
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Victor Maurizot et al.
Chemical communications (Cambridge, England), (8)(8), 924-925 (2004-04-08)
A heptameric amide of 2,6-diaminopyridine and 2,6-pyridinedicarboxylic acid coordinates to Cu(II) to assemble into a double helical complex with a string of six shortly spaced Cu ions.
Aurélie Guenet et al.
Chemistry (Weinheim an der Bergstrasse, Germany), 17(23), 6443-6452 (2011-04-22)
The design, synthesis and structural characterisation, in solution, of two new molecular turnstiles based on Sn-porphyrin derivatives are described. The system is composed of a stator (5-(4-pyridyl)-10,15,20-triphenylporphyrin), a hinge (Sn(IV)) and a rotor (handle equipped with 2,6-pyridinedicarboxamide as a tridentate
Synthetic ion transporters can induce apoptosis by facilitating chloride anion transport into cells.
Sung-Kyun Ko et al.
Nature chemistry, 6(10), 885-892 (2014-09-23)
Anion transporters based on small molecules have received attention as therapeutic agents because of their potential to disrupt cellular ion homeostasis. However, a direct correlation between a change in cellular chloride anion concentration and cytotoxicity has not been established for
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service