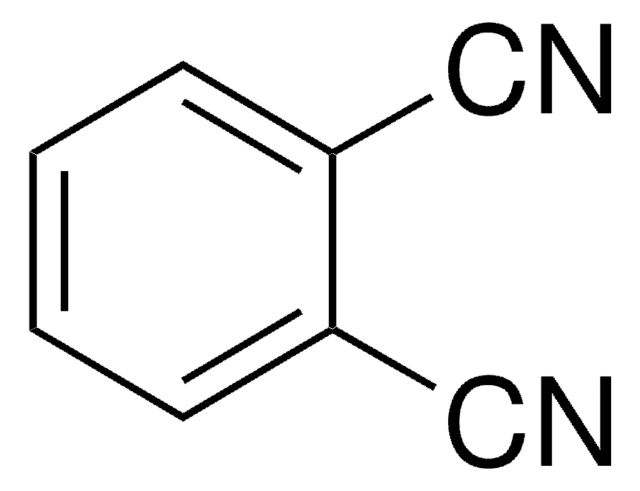
464899
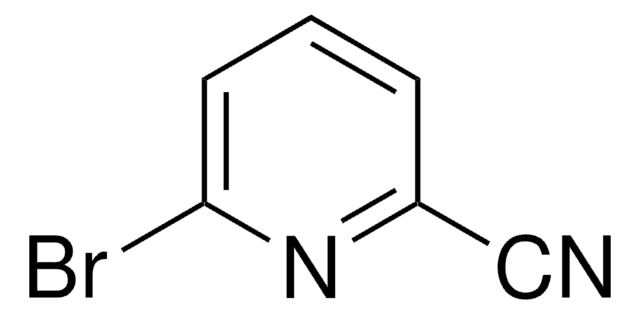
2,6-Pyridinedicarbonitrile
97%
Synonym(s):
2,6-Dicyanopyridine
Sign Into View Organizational & Contract Pricing
All Photos(3)
About This Item
Empirical Formula (Hill Notation):
C7H3N3
CAS Number:
Molecular Weight:
129.12
EC Number:
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Quality Level
Assay
97%
form
solid
mp
123-127 °C (lit.)
functional group
nitrile
SMILES string
N#Cc1cccc(n1)C#N
InChI
1S/C7H3N3/c8-4-6-2-1-3-7(5-9)10-6/h1-3H
InChI key
XNPMXMIWHVZGMJ-UHFFFAOYSA-N
General description
2,6-Pyridinedicarbonitrile is a heterocyclic dinitrile. Its biotransformation by Rhodococcus erythropolis A4 to 6-cyanopyridine-2-carboxamide has been reported.
Application
2,6-Pyridinedicarbonitrile may be used to synthesize bis-tetrazoles and pyridine-based tridentate ligand 2,6-bis(α-aminoisopropyl)pyridine.
Signal Word
Warning
Hazard Statements
Precautionary Statements
Hazard Classifications
Acute Tox. 4 Dermal - Acute Tox. 4 Inhalation - Acute Tox. 4 Oral - Eye Irrit. 2 - Skin Irrit. 2 - STOT SE 3
Target Organs
Respiratory system
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Synthesis and exploratory coordination chemistry of the new ditertiary carbinamine ligand 2, 6-bis (a-aminoisopropyl) pyridine.
Dahlenburg L, et al.
Inorgorganica Chimica Acta, 360(5), 1474-1481 (2007)
Vojtech Vejvoda et al.
Biotechnology letters, 29(7), 1119-1124 (2007-05-05)
2,6-Pyridinedicarbonitrile (1a) and 2,4-pyridinedicarbonitrile (2a) were hydrated by Rhodococcus erythropolis A4 to 6-cyanopyridine-2-carboxamide (1b; 83% yield) and 2-cyanopyridine-4-carboxamide (2b; 97% yield), respectively, after 10 min. After 118 h, the intermediates 1b or 2b were transformed into 2,6-pyridinedicarboxamide (1c; 35% yield)
Synthesis and characterisation of macrocycles containing both tetrazole and pyridine functionalities.
Fleming A, et al.
Tetrahedron, 67(18), 3260-3266 (2011)
Jonita Stankevičiūtė et al.
Scientific reports, 6, 39129-39129 (2016-12-17)
Pyridinols and pyridinamines are important intermediates with many applications in chemical industry. The pyridine derivatives are in great demand as synthons for pharmaceutical products. Moreover, pyridines are used either as biologically active substances or as building blocks for polymers with
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service




![1,8-Diazabicyclo[5.4.0]undec-7-ene 98%](/deepweb/assets/sigmaaldrich/product/structures/120/564/5b373e23-1624-489c-8efb-692de0f96ffb/640/5b373e23-1624-489c-8efb-692de0f96ffb.png)