232386
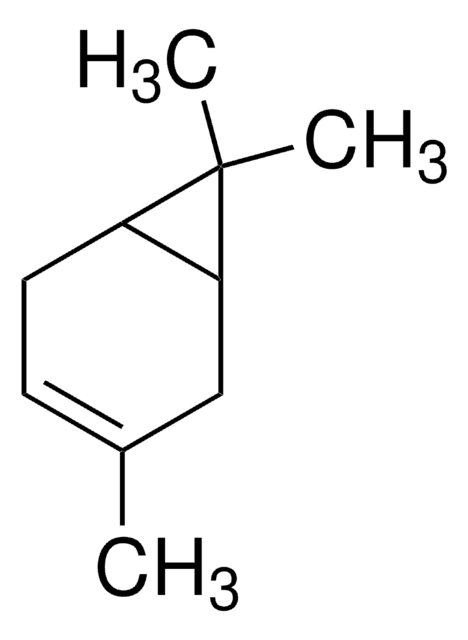
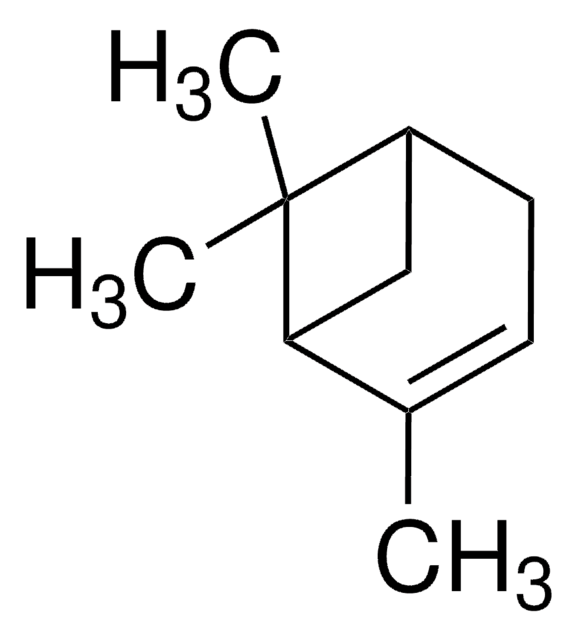
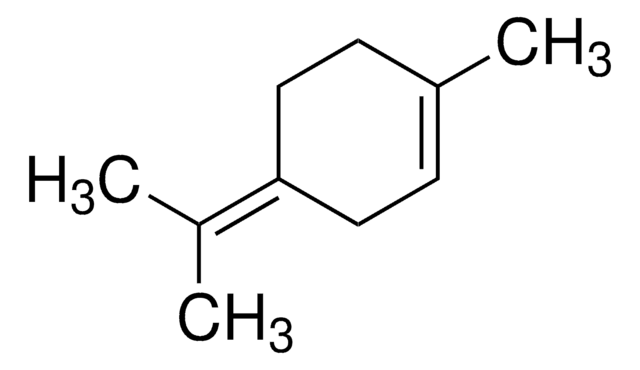
(+)-2-Carene
97%
Synonym(s):
(1S)-3,7,7-Trimethylbicyclo[4.1.0]hept-2-ene
Sign Into View Organizational & Contract Pricing
All Photos(1)
About This Item
Empirical Formula (Hill Notation):
C10H16
CAS Number:
Molecular Weight:
136.23
Beilstein:
2038651
EC Number:
MDL number:
UNSPSC Code:
12352002
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Quality Level
Assay
97%
optical activity
[α]20/D +90.0°, c = 6 in ethanol
refractive index
n20/D 1.476 (lit.)
bp
167-168 °C (lit.)
density
0.862 g/mL at 25 °C (lit.)
SMILES string
CC1=C[C@H]2[C@@H](CC1)C2(C)C
InChI
1S/C10H16/c1-7-4-5-8-9(6-7)10(8,2)3/h6,8-9H,4-5H2,1-3H3/t8-,9+/m1/s1
InChI key
IBVJWOMJGCHRRW-BDAKNGLRSA-N
Looking for similar products? Visit Product Comparison Guide
Related Categories
General description
(+)-2-Carene is a natural bicyclic monoterpene that is commonly found in the essential oils of various plants, such as rosemary, cedarwood, and pine. In organic synthesis, it is used as a chiral building block for the synthesis of a variety of chiral compounds.
Application
(+)-2-Carene can be used as a chiral building block in the synthesis of chiral non-racemic 2,2-dimethyl-1,3-disubstituted cyclopropane derivatives. It is also used as a reactant in the enantio-selective synthesis of (+)-α-elemene.
Signal Word
Warning
Hazard Statements
Precautionary Statements
Hazard Classifications
Flam. Liq. 3
Storage Class Code
3 - Flammable liquids
WGK
WGK 3
Flash Point(F)
100.4 °F - closed cup
Flash Point(C)
38 °C - closed cup
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Codruta Ignea et al.
Nature communications, 10(1), 3799-3799 (2019-08-25)
Synthetic biology efforts for the production of valuable chemicals are frequently hindered by the structure and regulation of the native metabolic pathways of the chassis. This is particularly evident in the case of monoterpenoid production in Saccharomyces cerevisiae, where the
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service