All Photos(1)
About This Item
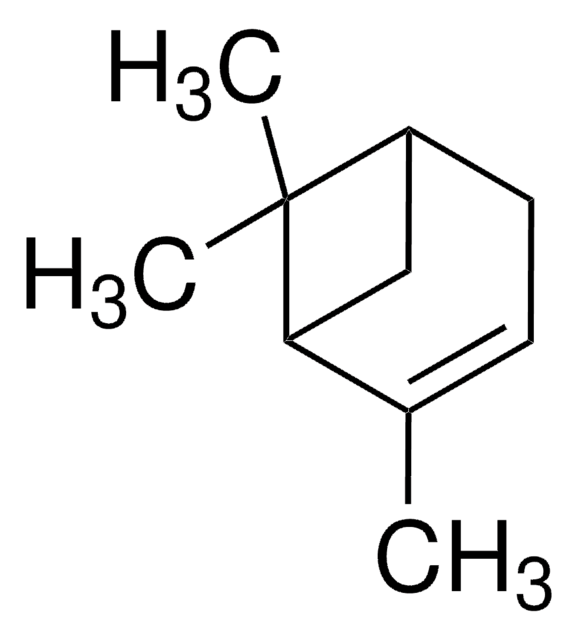
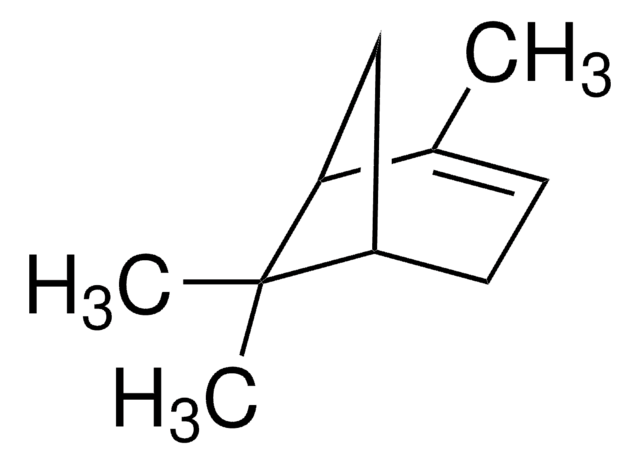
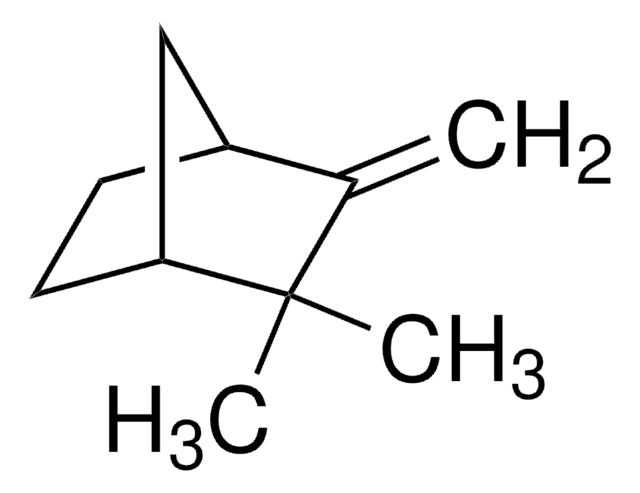
Empirical Formula (Hill Notation):
C10H16O
CAS Number:
Molecular Weight:
152.23
EC Number:
MDL number:
UNSPSC Code:
12162002
PubChem Substance ID:
NACRES:
NA.23
Recommended Products
Quality Level
Assay
97%
form
liquid
refractive index
n20/D 1.469 (lit.)
bp
102-103 °C/50 mmHg (lit.)
density
0.964 g/mL at 25 °C (lit.)
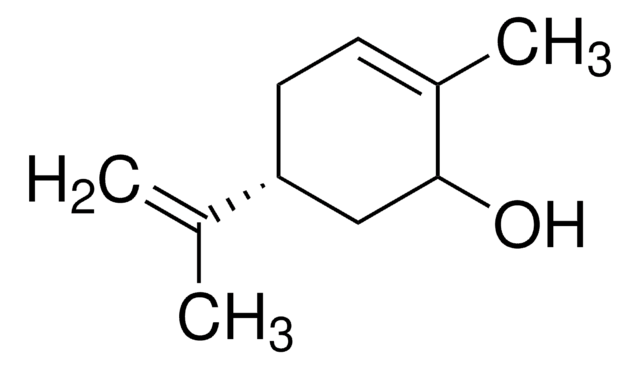
SMILES string
CC1(C)[C@H]2CC3OC3(C)[C@@H]1C2
InChI
1S/C10H16O/c1-9(2)6-4-7(9)10(3)8(5-6)11-10/h6-8H,4-5H2,1-3H3/t6-,7-,8?,10?/m1/s1
InChI key
NQFUSWIGRKFAHK-BGPATTHWSA-N
Related Categories
Storage Class Code
10 - Combustible liquids
WGK
WGK 3
Flash Point(F)
150.8 °F - closed cup
Flash Point(C)
66 °C - closed cup
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Denis Linares et al.
Bioresource technology, 99(11), 4590-4596 (2007-09-15)
The feasibility of trans-2-methyl-5-isopropylhexa-2,5-dienoic acid (novalic acid) accumulation using the alpha-pinene degradation pathway of Pseudomonas rhodesiae CIP 107491 was studied. This appeared possible by using concentrated living bacterial cells produced under oxygen limitation with alpha-pinene as sole carbon source. The
H Zorn et al.
Journal of biotechnology, 107(3), 255-263 (2004-01-23)
When submerged cultured Pseudomonas fluorescens NCIMB 11761 was fed-batch supplemented with alpha-pinene oxide, a rapid formation of 2,6-dimethyl-5-methylene-hept-(2Z)-enal (I) (isonovalal) was observed. Biotransformation and isomerisation of (I) to the (2E)-isomer (II) (novalal) were enhanced by Lewatit OC 1064, a macroporous
A Boontawan et al.
Applied microbiology and biotechnology, 69(6), 643-649 (2005-08-10)
In this work the biotransformation of alpha-pinene oxide to isonovalal using resting cells of Pseudomonas fluorescens NCIMB 11671 was evaluated in a membrane bioreactor for biotransformations (MBB). Since the membrane area required to obtain optimum productivities was calculated to be
Apichat Boontawan et al.
Biotechnology progress, 21(6), 1680-1687 (2005-12-03)
Terpenoids are important compounds for the fragrance industry, and recently, biocatalytic methods have been developed to produce them from cheap monoterpenes, such as alpha-pinene oxide. The biotransformation of alpha-pinene oxide using resting cells of Pseudomonas fluorescens NCIMB 11671 produces isonovalal
E T Griffiths et al.
Journal of bacteriology, 169(11), 4972-4979 (1987-11-01)
Over 20 gram-positive bacteria were isolated by elective culture with (+/-)-alpha-pinene as the sole carbon source. One of these strains, Nocardia sp. strain P18.3, was selected for detailed study. alpha-Pinene-grown cells oxidized, without lag, alpha-pinene, alpha-pinene oxide (epoxide), and the
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service