224448
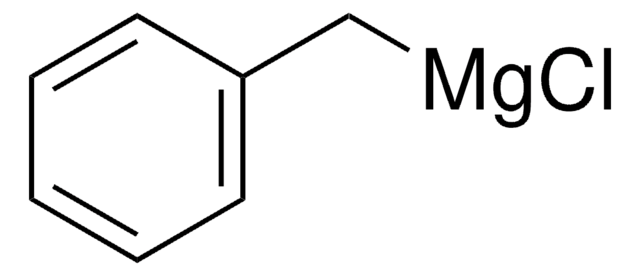
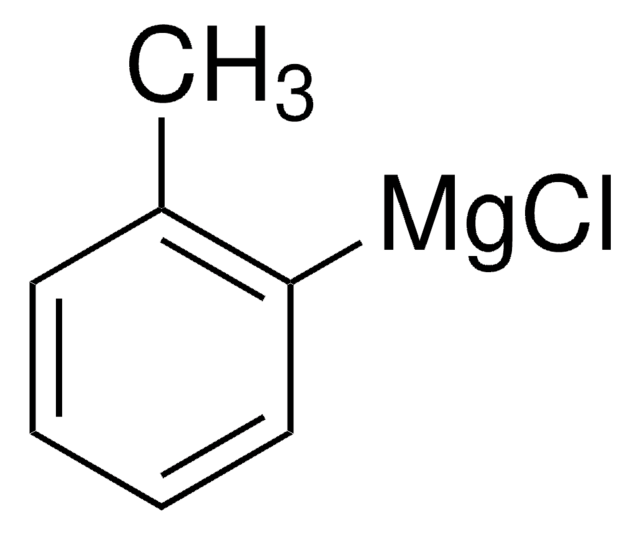
Phenylmagnesium chloride solution
2.0 M in THF
Synonym(s):
Chlorophenylmagnesium
About This Item
Recommended Products
Quality Level
reaction suitability
reaction type: Grignard Reaction
concentration
2.0 M in THF
density
~1.02 g/mL at 20 °C
1.042 g/mL at 25 °C
SMILES string
Cl[Mg]c1ccccc1
InChI
1S/C6H5.ClH.Mg/c1-2-4-6-5-3-1;;/h1-5H;1H;/q;;+1/p-1
InChI key
GQONLASZRVFGHI-UHFFFAOYSA-M
Looking for similar products? Visit Product Comparison Guide
Related Categories
Application
Packaging
Other Notes
Signal Word
Danger
Hazard Statements
Precautionary Statements
Hazard Classifications
Carc. 2 - Eye Dam. 1 - Flam. Liq. 2 - Skin Corr. 1B - STOT SE 3
Target Organs
Central nervous system, Respiratory system
Supplementary Hazards
Storage Class Code
3 - Flammable liquids
WGK
WGK 3
Flash Point(F)
1.4 °F - closed cup
Flash Point(C)
-17 °C - closed cup
Personal Protective Equipment
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Articles
We carry a large variety of electrophiles and nucleophiles that are widely used in C–C bond-forming reactions. This group of products contains many organometallic reagents as well as commonly-used alkylating and acylating reagents.
Global Trade Item Number
| SKU | GTIN |
|---|---|
| 224448-18L | |
| 224448-800ML | 4061838779045 |
| 224448-8L | 4061838125712 |
| 224448-100ML | 4061838779038 |
| 224448-18L-C | |
| 224448-18L-KL | 4061838135032 |
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service