171565
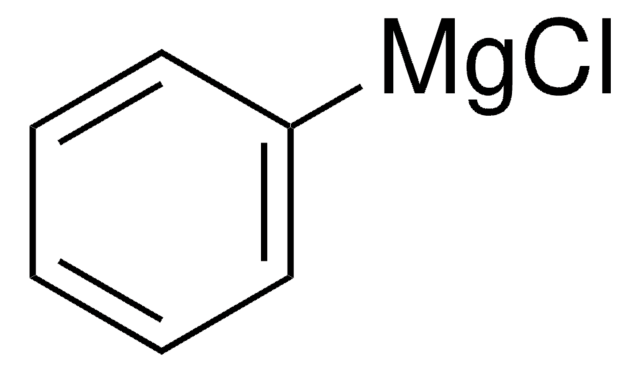
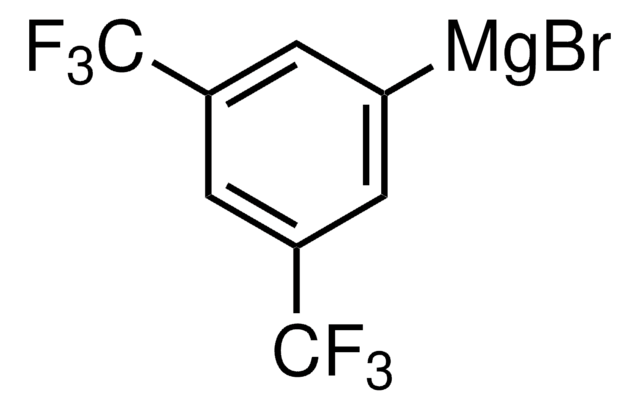
Phenylmagnesium bromide solution
3.0 M in diethyl ether
Synonym(s):
Bromomagnesiobenzene, Bromophenylmagnesium
About This Item
Recommended Products
Quality Level
reaction suitability
reaction type: Grignard Reaction
concentration
3.0 M in diethyl ether
density
1.134 g/mL at 25 °C
SMILES string
Br[Mg]c1ccccc1
InChI
1S/C6H5.BrH.Mg/c1-2-4-6-5-3-1;;/h1-5H;1H;/q;;+1/p-1
InChI key
ANRQGKOBLBYXFM-UHFFFAOYSA-M
Looking for similar products? Visit Product Comparison Guide
Related Categories
General description
Application
It may be used for synthesis of the following:
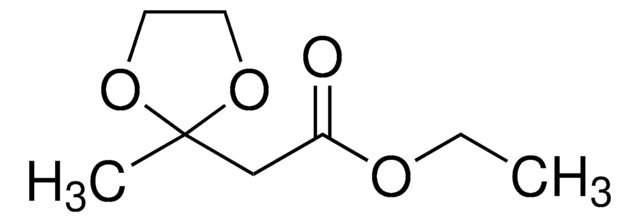
- 1,3,3-trimethyl-6-phenyl-2-oxabicyclo[2.2.2]octan-6-ol
- 6-benzyl-1,3,3-trimethyl-2-oxabicyclo[2.2.2]octan-6-ol
- (3-(2-Dithiobenzoatepropionyl)propyl)dimethylmethoxysilane, reversible addition-fragmentation chain transfer polymerization (RAFT)-silane agent
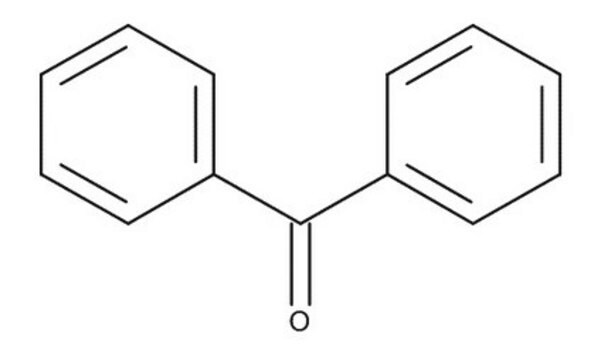
- series of o-substituted benzophenones
Packaging
Other Notes
Signal Word
Danger
Hazard Statements
Precautionary Statements
Hazard Classifications
Eye Dam. 1 - Flam. Liq. 2 - Skin Corr. 1B - STOT SE 3
Target Organs
Respiratory system
Supplementary Hazards
Storage Class Code
3 - Flammable liquids
WGK
WGK 3
Flash Point(F)
-40.0 °F - closed cup
Flash Point(C)
-40 °C - closed cup
Personal Protective Equipment
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Articles
We carry a large variety of electrophiles and nucleophiles that are widely used in C–C bond-forming reactions. This group of products contains many organometallic reagents as well as commonly-used alkylating and acylating reagents.
Global Trade Item Number
| SKU | GTIN |
|---|---|
| S892858-1EA | |
| 171565-18L | 4061838751065 |
| 171565-50ML | 4061838751072 |
| 171565-800ML | 4061838751089 |
| 171565-100ML | 4061838751058 |
| 171565-18L-C | 4061838234902 |
| 171565-4X25ML | 4065268444450 |
| 171565-8L |
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service