1A00550
USP
(R)-CLOPIDOGREL CARBOXYLIC ACID
Pharmaceutical Analytical Impurity (PAI)
Sinônimo(s):
(R)-2-(2-chlorophenyl)-2-(6,7-dihydrothieno[3,2-c]pyridin-5(4H)-yl)acetic acid
Faça loginpara ver os preços organizacionais e de contrato
About This Item
Código UNSPSC:
12352106
NACRES:
NA.24
Produtos recomendados
grau
pharmaceutical analytical impurity (PAI)
Agency
USP
família API
clopidogrel
fabricante/nome comercial
USP
aplicação(ões)
pharmaceutical
Formato
neat
temperatura de armazenamento
2-8°C
Descrição geral
(R)-CLOPIDOGREL CARBOXYLIC ACID is a USP Pharmaceutical Analytical Impurity (PAI).
USP PAI are a product line of impurities suitable for research and analytical purposes, which help to ensure the quality and safety of medicines.
Associated Drug Substance: Clopidogrel Bisulfate
Therapeutic Area: Cardiovascular
For more information about this PAI, visit here.
USP PAI are a product line of impurities suitable for research and analytical purposes, which help to ensure the quality and safety of medicines.
Associated Drug Substance: Clopidogrel Bisulfate
Therapeutic Area: Cardiovascular
For more information about this PAI, visit here.
Aplicação
(R)-CLOPIDOGREL CARBOXYLIC ACID (USP PAI) is intended for use in analytical testing to detect, identify, and measure pharmaceutical impurities.
Características e benefícios
USP PAI advance your early analytical R&D and process development. PAI can be used in the following applications:
1. Conduct analytical tests during early formulation feasibility studies.
2. Determine degradation impurities produced during stress studies.
3. Develop, validate, and transfer analytical methods.
4. Perform spiking studies during process R&D to demonstrate depletion upon recrystallization.
5. Record retention times and/or spectra
6. Determine relative response factors.
7. Identify unknown impurities that formed during ICH stability conditions.
8. Identify impurities that are present in the Reference Listed Drug
9. Test for and profile impurities not listed in drug substance and drug product monographs.
1. Conduct analytical tests during early formulation feasibility studies.
2. Determine degradation impurities produced during stress studies.
3. Develop, validate, and transfer analytical methods.
4. Perform spiking studies during process R&D to demonstrate depletion upon recrystallization.
5. Record retention times and/or spectra
6. Determine relative response factors.
7. Identify unknown impurities that formed during ICH stability conditions.
8. Identify impurities that are present in the Reference Listed Drug
9. Test for and profile impurities not listed in drug substance and drug product monographs.
Nota de análise
These products are for test and assay use only. They are not meant for administration to humans or animals and cannot be used to diagnose, treat, or cure diseases of any kind.
Outras notas
Sales restrictions may apply.
Código de classe de armazenamento
11 - Combustible Solids
Classe de risco de água (WGK)
WGK 3
Ponto de fulgor (°F)
Not applicable
Ponto de fulgor (°C)
Not applicable
Escolha uma das versões mais recentes:
Certificados de análise (COA)
Lot/Batch Number
It looks like we've run into a problem, but you can still download Certificates of Analysis from our Documentos section.
Se precisar de ajuda, entre em contato Atendimento ao cliente
Já possui este produto?
Encontre a documentação dos produtos que você adquiriu recentemente na biblioteca de documentos.
Nossa equipe de cientistas tem experiência em todas as áreas de pesquisa, incluindo Life Sciences, ciência de materiais, síntese química, cromatografia, química analítica e muitas outras.
Entre em contato com a assistência técnica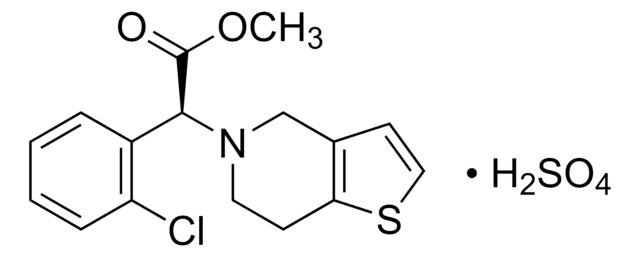



![2-Hydroxy-N-[(2R)-2-hydroxy-2-(3-hydroxyphenyl)ethyl]-N-methylacetamide Pharmaceutical Secondary Standard; Certified Reference Material](/deepweb/assets/sigmaaldrich/product/images/944/633/33394fd6-b6f6-4766-8c7e-1c6650967ee2/640/33394fd6-b6f6-4766-8c7e-1c6650967ee2.jpg)