P0440
Pimaricin preparation
~2.5% (γ-irradiated Pimaricin), aqueous suspension
Sinônimo(s):
Pimaricin, Tennecetin, Natamycin preparation
About This Item
forma
aqueous suspension
concentração
~2.5% (γ-irradiated Pimaricin)
solubilidade
DMSO: soluble
densidade
1.0 g/mL at 20 °C (lit.)
espectro de atividade do antibiótico
fungi
yeast
Modo de ação
cell membrane | interferes
temperatura de armazenamento
2-8°C
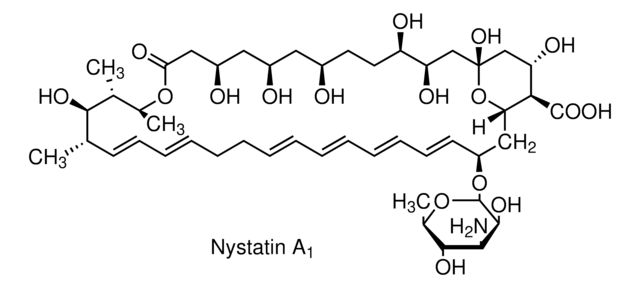
cadeia de caracteres SMILES
[H][C@]12C[C@@H](O[C@@H]3O[C@H](C)[C@@H](O)[C@H](N)[C@@H]3O)\C=C\C=C\C=C\C=C\C[C@@H](C)OC(=O)\C=C\[C@@]4([H])O[C@]4([H])C[C@H](O)C[C@](O)(C[C@H](O)[C@H]1C(O)=O)O2
InChI
1S/C33H47NO13/c1-18-10-8-6-4-3-5-7-9-11-21(45-32-30(39)28(34)29(38)19(2)44-32)15-25-27(31(40)41)22(36)17-33(42,47-25)16-20(35)14-24-23(46-24)12-13-26(37)43-18/h3-9,11-13,18-25,27-30,32,35-36,38-39,42H,10,14-17,34H2,1-2H3,(H,40,41)/b4-3+,7-5+,8-6+,11-9+,13-12+/t18-,19-,20+,21+,22+,23-,24-,25+,27-,28+,29-,30+,32+,33-/m1/s1
chave InChI
NCXMLFZGDNKEPB-FFPOYIOWSA-N
Procurando produtos similares? Visita Guia de comparação de produtos
Categorias relacionadas
Descrição geral
Pimaricin is a polyene antifungal antibiotic produced by Streptomyces natalensis from soil near Pietermaritzburg, South Africa.1 Pimaricin has antimicrobial activity similar to that of nystatin. In addition, it is active against Trichomonas vaginalis. Pimaricin is used in the treatment of candidiasis, trichomoniasis, fungal keratitis and aspergillosis. It has also been used as a food additive in some countries. In some studies, it has been shown to decrease the amount of mold upon which the Dermatophagoides pteronyssinus (house-dust mite) is dependent.2
Aplicação
Ações bioquímicas/fisiológicas
Nota de preparo
The product and any aqueous dilutions will be suspensions and should not be sterile filtered.
Armazenamento e estabilidade
Outras notas
Código de classe de armazenamento
10 - Combustible liquids
Classe de risco de água (WGK)
WGK 2
Ponto de fulgor (°F)
Not applicable
Ponto de fulgor (°C)
Not applicable
Equipamento de proteção individual
Eyeshields, Gloves
Certificados de análise (COA)
Busque Certificados de análise (COA) digitando o Número do Lote do produto. Os números de lote e remessa podem ser encontrados no rótulo de um produto após a palavra “Lot” ou “Batch”.
Já possui este produto?
Encontre a documentação dos produtos que você adquiriu recentemente na biblioteca de documentos.
Os clientes também visualizaram
Nossa equipe de cientistas tem experiência em todas as áreas de pesquisa, incluindo Life Sciences, ciência de materiais, síntese química, cromatografia, química analítica e muitas outras.
Entre em contato com a assistência técnica