31581
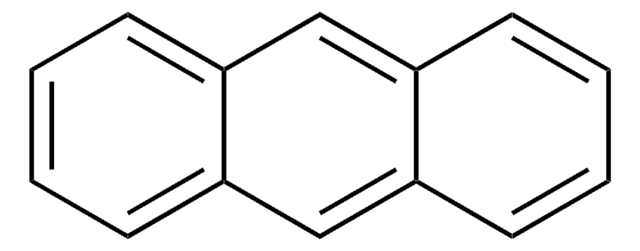
Anthracene
analytical standard
Sinônimo(s):
Anthraxcene, Paranaphthalene
About This Item
Produtos recomendados
grau
analytical standard
Nível de qualidade
densidade de vapor
6.15 (vs air)
pressão de vapor
1 mmHg ( 145 °C)
temperatura de autoignição
1004 °F
prazo de validade
limited shelf life, expiry date on the label
técnica(s)
HPLC: suitable
gas chromatography (GC): suitable
p.e.
340 °C (lit.)
pf
210-215 °C (lit.)
solubilidade
alcohols: soluble
benzene: soluble
chloroform: soluble
hydronaphthalenes: soluble
supercritical carbon dioxide: soluble
aplicação(ões)
environmental
Formato
neat
cadeia de caracteres SMILES
c1ccc2cc3ccccc3cc2c1
InChI
1S/C14H10/c1-2-6-12-10-14-8-4-3-7-13(14)9-11(12)5-1/h1-10H
chave InChI
MWPLVEDNUUSJAV-UHFFFAOYSA-N
Procurando produtos similares? Visita Guia de comparação de produtos
Descrição geral
Aplicação
Produtos recomendados
Palavra indicadora
Warning
Frases de perigo
Declarações de precaução
Classificações de perigo
Aquatic Acute 1 - Aquatic Chronic 1 - Eye Irrit. 2
Código de classe de armazenamento
11 - Combustible Solids
Classe de risco de água (WGK)
WGK 2
Ponto de fulgor (°F)
249.8 °F - closed cup
Ponto de fulgor (°C)
121.0 °C - closed cup
Equipamento de proteção individual
dust mask type N95 (US), Eyeshields, Gloves
Escolha uma das versões mais recentes:
Certificados de análise (COA)
Não está vendo a versão correta?
Se precisar de uma versão específica, você pode procurar um certificado específico pelo número do lote ou da remessa.
Já possui este produto?
Encontre a documentação dos produtos que você adquiriu recentemente na biblioteca de documentos.
Os clientes também visualizaram
Protocolos
GC Analysis of PAHs on SLB®-5ms
US EPA Method 610 describes the analysis of polynuclear aromatic hydrocarbons (commonly referred to as PAHs or PNAs) by both HPLC and GC.
HPLC Analysis of PAHs on SUPELCOSIL™ LC-PAH
GC Analysis of Polynuclear Aromatic Hydrocarbons (PAHs) in Salmon on SPB®-608 (20 m x 0.18 mm I.D., 0.18 µm) after QuEChERS Cleanup using Supel™ QuE Z-Sep, Fast GC Analysis
Nossa equipe de cientistas tem experiência em todas as áreas de pesquisa, incluindo Life Sciences, ciência de materiais, síntese química, cromatografia, química analítica e muitas outras.
Entre em contato com a assistência técnica