700080P
Avanti
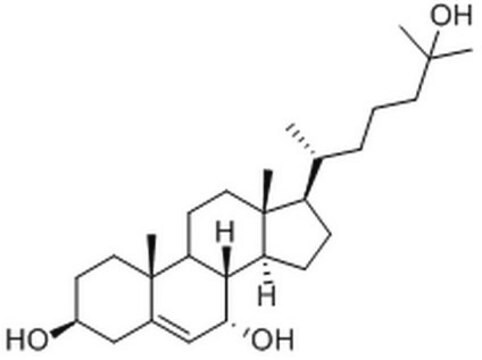
7α,25-dihydroxycholesterol
Avanti Research™ - A Croda Brand
Sinônimo(s):
cholest-5-ene-3β,7α,25-triol
Faça loginpara ver os preços organizacionais e de contrato
About This Item
Fórmula empírica (Notação de Hill):
C27H46O3
Número CAS:
Peso molecular:
418.65
Código UNSPSC:
12352211
NACRES:
NA.25
Produtos recomendados
forma
powder
embalagem
pkg of 1 × 1 mg (700080P-1mg)
fabricante/nome comercial
Avanti Research™ - A Croda Brand
Condições de expedição
dry ice
temperatura de armazenamento
−20°C
Descrição geral
7α,25-dihydroxycholesterol (7α,25-OHC) is synthesized by the hydroxylation of cholesterol by the action of enzyme cholesterol 25-hydroxylase (CH25H) and cytochrome P450, family 7, subfamily b, polypeptide 1 (CYP7B1). The catabolic breakdown of 7α,25-OHC to bile acid precursors is catalyzed by the enzymes hydroxy-Δ-5-steroid dehydrogenase, 3β- and steroid Δ-isomerase 7 (HSD3B7).
Aplicação
7α,25-dihydroxycholesterol has been used:
- as a G-protein-coupled receptor 183 ( GPR183) ligand in chemotaxis assay and intracellular cytokine staining method and in fluorescence-activated cell sorting (FACS) analysis of natural killer cells
- in oxysterol based calcium mobilization assay in Chinese hamster ovary (CHO) cells
- in competitive radioligand binding assay of Epstein-Barr virus-induced gene 2 (EBI2) expressing COS-7 cells
Ações bioquímicas/fisiológicas
7α,25-dihydroxycholesterol (7α,25-OHC) serves as an endogenous ligand for G-protein-coupled receptor 183 (GPR183) or Epstein-Barr virus-induced gene 2 (EBI2). It plays a key role in the migration of type 3 innate lymphoid cells (ILC3) in the small intestine and colon. 7α,25-OHC mediates immune cell migration functionality by their chemoattractant property. Inhibition of 7α,25-OHC synthesis leads to impairment in the migration of B cells.
Embalagem
5 mL Amber Glass Screw Cap Vial (700080P-1mg)
Informações legais
Avanti Research is a trademark of Avanti Polar Lipids, LLC
geralmente comprado junto com este produto
Nº do produto
Descrição
Preços
Código de classe de armazenamento
11 - Combustible Solids
Classe de risco de água (WGK)
WGK 3
Ponto de fulgor (°F)
Not applicable
Ponto de fulgor (°C)
Not applicable
Certificados de análise (COA)
Busque Certificados de análise (COA) digitando o Número do Lote do produto. Os números de lote e remessa podem ser encontrados no rótulo de um produto após a palavra “Lot” ou “Batch”.
Já possui este produto?
Encontre a documentação dos produtos que você adquiriu recentemente na biblioteca de documentos.
Gene regulatory programs conferring phenotypic identities to human NK cells
Collins PL, et al.
Cell, 176(1-2), 348-360 (2019)
Identification of structural motifs critical for epstein-barr virus-induced molecule 2 function and homology modeling of the ligand docking site
Zhang L, et al.
Molecular Pharmacology, 82(6), 1094-1103 (2012)
Katharina R Beck et al.
The Journal of steroid biochemistry and molecular biology, 190, 19-28 (2019-03-25)
Oxysterols are cholesterol metabolites derived through either autoxidation or enzymatic processes. They consist of a large family of bioactive lipids that have been associated with the progression of multiple pathologies. In order to unravel (patho-)physiological mechanisms involving oxysterols, it is
Nossa equipe de cientistas tem experiência em todas as áreas de pesquisa, incluindo Life Sciences, ciência de materiais, síntese química, cromatografia, química analítica e muitas outras.
Entre em contato com a assistência técnica