93093
Triphenylphosphine, polymer-bound
100-200 mesh, extent of labeling: ~3 mmol/g triphenylphosphine loading
Sinônimo(s):
Copolymer of styrene and divinylbenzene, diphenylphosphinated, Diphenylphosphino-polystyrene, Polystyrene crosslinked with divinylbenzene, diphenylphosphinated
About This Item
Produtos recomendados
Formulário
solid
Nível de qualidade
adequação da reação
reaction type: Buchwald-Hartwig Cross Coupling Reaction
reaction type: Heck Reaction
reaction type: Hiyama Coupling
reaction type: Negishi Coupling
reaction type: Sonogashira Coupling
reaction type: Stille Coupling
reaction type: Suzuki-Miyaura Coupling
reaction type: solution phase peptide synthesis
reagent type: ligand
Extensão da rotulagem
~3 mmol/g triphenylphosphine loading
Matriz
crosslinked with 2% DVB
tamanho de partícula
100-200 mesh
grupo funcional
phosphine oxide
cadeia de caracteres SMILES
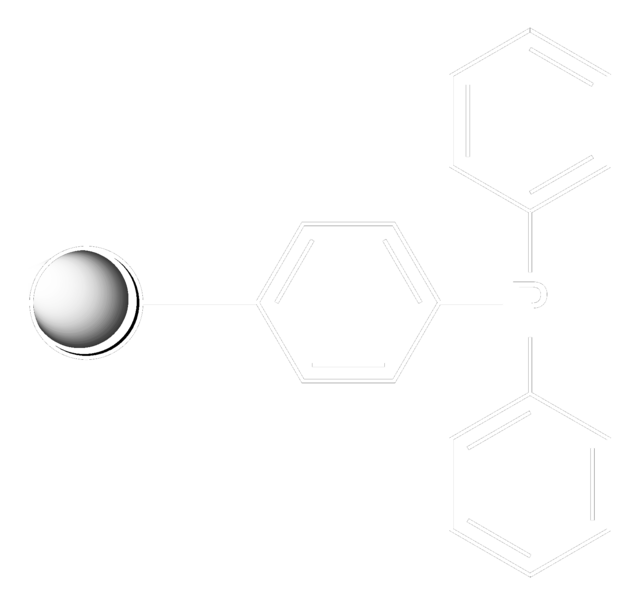
c1ccc(cc1)P(c2ccccc2)c3ccccc3
InChI
1S/C18H15P/c1-4-10-16(11-5-1)19(17-12-6-2-7-13-17)18-14-8-3-9-15-18/h1-15H
chave InChI
RIOQSEWOXXDEQQ-UHFFFAOYSA-N
Procurando produtos similares? Visita Guia de comparação de produtos
Aplicação
It can also be used:
- For the esterification of alkylphosphonic acids using primary alcohols in the presence of iodine and imidazole.
- To catalyze the conversion of 3-[3-(1,3-dioxolan-2-yl)-1-hydroxypropyl]pyridine to 3-[1-bromo-3-(1,3-dioxolan-2-yl)propyl]pyridine using carbon tetrabromide.
- To isomerize (Z)-nitro olefins to the (E)-isomers.
- To prepare polymer-bound ylides which are useful in Wittig reactions.
- To convert alcohols or carboxylic acids to the corresponding chlorides.
- In combination with carbon tetrachloride for the coupling N-alkoxycarbonyl α-amino acids and primary amines to form the corresponding amides.
Outras notas
Código de classe de armazenamento
11 - Combustible Solids
Classe de risco de água (WGK)
WGK 3
Equipamento de proteção individual
dust mask type N95 (US), Eyeshields, Faceshields, Gloves
Escolha uma das versões mais recentes:
Já possui este produto?
Encontre a documentação dos produtos que você adquiriu recentemente na biblioteca de documentos.
Nossa equipe de cientistas tem experiência em todas as áreas de pesquisa, incluindo Life Sciences, ciência de materiais, síntese química, cromatografia, química analítica e muitas outras.
Entre em contato com a assistência técnica