417130
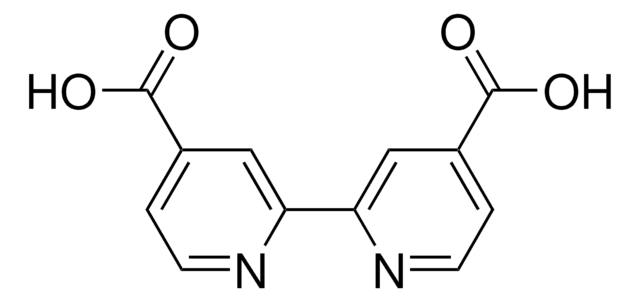
Benzene-1,4-diboronic acid
≥95.0%
Sinônimo(s):
p-Phenylenediboronic acid, 1,4-Phenylenebisboronic acid, 1,4-Phenylenediboronic acid, p-Benzenediboronic acid, Benzene-1,4-diboronic acid, NSC 25410
About This Item
Produtos recomendados
Nível de qualidade
Ensaio
≥95.0%
Formulário
powder
pf
>350 °C (lit.)
cadeia de caracteres SMILES
OB(O)c1ccc(cc1)B(O)O
InChI
1S/C6H8B2O4/c9-7(10)5-1-2-6(4-3-5)8(11)12/h1-4,9-12H
chave InChI
BODYVHJTUHHINQ-UHFFFAOYSA-N
Procurando produtos similares? Visita Guia de comparação de produtos
Categorias relacionadas
Aplicação
- Externally initiated Kumada catalyst-transfer polycondensation
- Suzuki-Miyaura cross-coupling reactions
- Energy transfer processes in optoelectronic devices
- Palladium-catalyzed sequential alkenylation and conjugate addition reactions
- Scholl cyclizations
Reagent used in Preparation of
- Crosslinkers and cross-linked core-shell nanoparticles by RAFT polymerization and palladium-catalyzed Suzuki coupling reaction
- Fluorescence and solution-processable coordination polymers
- Cyclotricatechylene based porous crystalline material for gas storage
- Indolizine derivatives as OLEDs
- Helically p-stacked thiophene-based copolymers with circularly polarized fluorescence
- Novel organic semiconductors and applications in organic thin-film transistors
- Highly twisted polycyclic aromatic hydrocarbons with unexptected reactivity
Outras notas
Palavra indicadora
Warning
Frases de perigo
Declarações de precaução
Classificações de perigo
Acute Tox. 4 Oral
Código de classe de armazenamento
11 - Combustible Solids
Classe de risco de água (WGK)
WGK 3
Ponto de fulgor (°F)
Not applicable
Ponto de fulgor (°C)
Not applicable
Equipamento de proteção individual
dust mask type N95 (US), Eyeshields, Gloves
Escolha uma das versões mais recentes:
Já possui este produto?
Encontre a documentação dos produtos que você adquiriu recentemente na biblioteca de documentos.
Os clientes também visualizaram
Artigos
Professor Aran (Claremont University, USA) thoroughly discusses the engineering of graphene based materials through careful functionalization of graphene oxide, a solution processable form of graphene.
Global Trade Item Number
| SKU | GTIN |
|---|---|
| 417130-5G | 4061832090085 |
| 417130-25G | 4061832090078 |
Nossa equipe de cientistas tem experiência em todas as áreas de pesquisa, incluindo Life Sciences, ciência de materiais, síntese química, cromatografia, química analítica e muitas outras.
Entre em contato com a assistência técnica