C4706
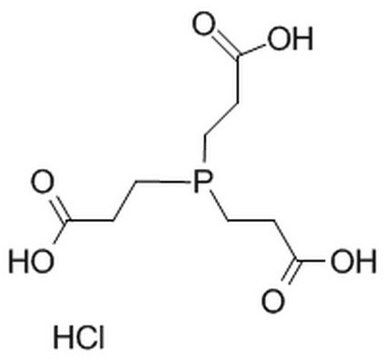
Tris(2-carboxyethyl)phosphine hydrochloride
powder
Synonym(s):
TCEP
Sign Into View Organizational & Contract Pricing
All Photos(3)
About This Item
Empirical Formula (Hill Notation):
C9H15O6P · HCl
CAS Number:
Molecular Weight:
286.65
Beilstein:
3724376
MDL number:
UNSPSC Code:
12352128
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
description
Protect from moisture
Quality Level
form
powder
reaction suitability
reagent type: reductant
color
white
solubility
H2O: 50 mg/mL
storage temp.
2-8°C
SMILES string
Cl[H].OC(=O)CCP(CCC(O)=O)CCC(O)=O
InChI
1S/C9H15O6P.ClH/c10-7(11)1-4-16(5-2-8(12)13)6-3-9(14)15;/h1-6H2,(H,10,11)(H,12,13)(H,14,15);1H
InChI key
PBVAJRFEEOIAGW-UHFFFAOYSA-N
Looking for similar products? Visit Product Comparison Guide
Related Categories
General description
Tris(2-carboxyethyl)phosphine hydrochloride (TCEP.HCl) is a non-volatile solid. It is a strong reducing agent.1 It can be synthesized by the acid hydrolysis of tris(2-cyanoethyl)phosphine in refluxing aqueous HCl.1 It has various biological applications such as in vitro and in vivo reduction of disulfide bonds in various peptides and proteins. TCEP is a useful chelating agent for various heavy metal ions as Zn(II), Cd(II), Pb(II), and Ni(II).
Application
Tris(2-carboxyethyl)phosphine hydrochloride (TCEP. HCl) can be used:
- As a reducing agent for the reduction of sulfoxides, sulfonyl chlorides, N-oxides, and azides. It can also be used in azide-alkyne cycloaddition reaction in the presence of a copper catalyst.
- To reduce disulfide bonds in various proteins.
- As a reagent for the selective reduction of disulfides in water.
- To remove ruthenium-derived metathesis catalysts via aqueous washing of a crude reaction mixture when it is basified.
- As a reducing agent for the reduction of various alkyl disulfides such as trans-4,5-dihydroxy-1,2-dithiane.
Water soluble reagent, used for selective reduction of disulfides. More stable than DTT and useful in mass spectrometry applications.
Caution
Storage conditions:protect from moisture.
related product
Signal Word
Danger
Hazard Statements
Precautionary Statements
Hazard Classifications
Eye Dam. 1 - Skin Corr. 1B
Storage Class Code
8A - Combustible corrosive hazardous materials
WGK
WGK 1
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Burns, J. A., et al.
The Journal of Organic Chemistry, 56, 1648-1648 (1991)
Selective reduction of disulfides by tris (2-carboxyethyl) phosphine.
Burns JA, et al.
The Journal of Organic Chemistry, 56(8), 2648-2650 (1991)
Coordination properties of tris (2-carboxyethyl) phosphine, a newly introduced thiol reductant, and its oxide.
Krezel A, et al.
Inorganic Chemistry, 42(6), 1994-2003 (2003)
Maynard, H. D.; Grubbs, R. J.
Tetrahedron Letters, 40, 4137-4137 (1999)
Andrea Krapp et al.
Cell reports, 26(4), 1044-1058 (2019-01-24)
Meiotic progression in S. pombe is regulated by stage-specific gene expression and translation, changes in RNA stability, expression of anti-sense transcripts, and targeted proteolysis of regulatory proteins. We have used SILAC labeling to examine the relative levels of proteins in diploid
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service