1242000
USP
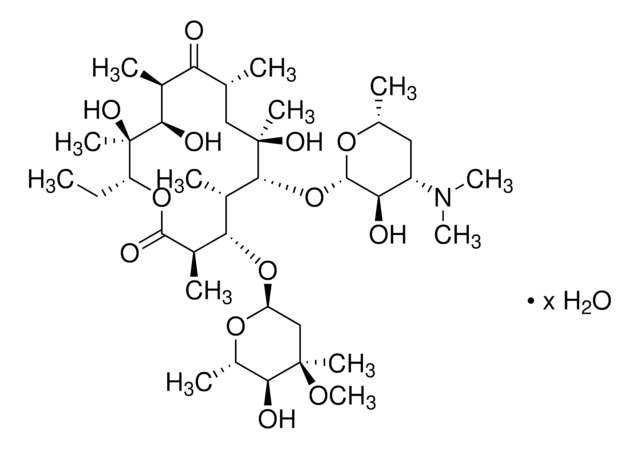
Erythromycin
United States Pharmacopeia (USP) Reference Standard
About This Item
Produits recommandés
Qualité
pharmaceutical primary standard
Famille d'API
erythromycin
Fabricant/nom de marque
USP
Application(s)
pharmaceutical (small molecule)
Format
neat
Température de stockage
−20°C
Chaîne SMILES
CC[C@H]1OC(=O)[C@H](C)[C@@H](O[C@H]2C[C@@](C)(OC)[C@@H](O)[C@H](C)O2)[C@H](C)[C@@H](O[C@@H]3O[C@H](C)C[C@@H]([C@H]3O)N(C)C)[C@](C)(O)C[C@@H](C)C(=O)[C@H](C)[C@@H](O)[C@]1(C)O
InChI
1S/C37H67NO13/c1-14-25-37(10,45)30(41)20(4)27(39)18(2)16-35(8,44)32(51-34-28(40)24(38(11)12)15-19(3)47-34)21(5)29(22(6)33(43)49-25)50-26-17-36(9,46-13)31(42)23(7)48-26/h18-26,28-32,34,40-42,44-45H,14-17H2,1-13H3/t18-,19-,20+,21+,22-,23+,24+,25-,26+,28-,29+,30-,31+,32-,34+,35-,36-,37-/m1/s1
Clé InChI
ULGZDMOVFRHVEP-RWJQBGPGSA-N
Vous recherchez des produits similaires ? Visite Guide de comparaison des produits
Description générale
Erythromycin USP reference standard is provided as delivered and specified by the issuing Pharmacopoeia. All information provided in support of this product, including MSDS and any product information leaflets, have been developed and issued under the Authority of the issuing Pharmacopoeia. For further information and support please go to the website of the issuing Pharmacopoeia.
Application
- Erythromycin Stearate
- Erythromycin Ethylsuccinate
- Erythromycin Lactobionate for Injection
- Erythromycin Ointment
- Erythromycin Pledgets
- Erythromycin Injection
Actions biochimiques/physiologiques
Antimicrobial Spectrum: This product acts against both gram-negative and gram-positive bacteria.
Attention
Notes préparatoires
Autres remarques
Informations légales
Produit(s) apparenté(s)
Code de la classe de stockage
11 - Combustible Solids
Classe de danger pour l'eau (WGK)
WGK 3
Point d'éclair (°F)
Not applicable
Point d'éclair (°C)
Not applicable
Certificats d'analyse (COA)
Recherchez un Certificats d'analyse (COA) en saisissant le numéro de lot du produit. Les numéros de lot figurent sur l'étiquette du produit après les mots "Lot" ou "Batch".
Déjà en possession de ce produit ?
Retrouvez la documentation relative aux produits que vous avez récemment achetés dans la Bibliothèque de documents.
Les clients ont également consulté
Notre équipe de scientifiques dispose d'une expérience dans tous les secteurs de la recherche, notamment en sciences de la vie, science des matériaux, synthèse chimique, chromatographie, analyse et dans de nombreux autres domaines..
Contacter notre Service technique