SML0186
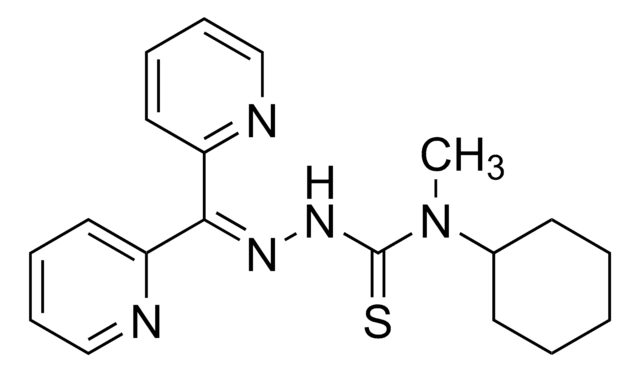
Dp44mT
≥98% (HPLC)
Synonyme(s) :
2-(Di-2-pyridinylmethylene)-N,N-dimethyl-hydrazinecarbothioamide, Di-2-pyridylketone-4,4,-dimethyl-3-thiosemicarbazone
About This Item
Produits recommandés
Niveau de qualité
Essai
≥98% (HPLC)
Forme
powder
Couleur
yellow to orange
Solubilité
DMSO: ≥5 mg/mL
Auteur
Bayer
Température de stockage
2-8°C
Chaîne SMILES
CN(C)C(=S)N\N=C(\c1ccccn1)c2ccccn2
InChI
1S/C14H15N5S/c1-19(2)14(20)18-17-13(11-7-3-5-9-15-11)12-8-4-6-10-16-12/h3-10H,1-2H3,(H,18,20)
Clé InChI
XOBIGRNRXCAMJQ-UHFFFAOYSA-N
Application
Actions biochimiques/physiologiques
Caractéristiques et avantages
Attention
Mention d'avertissement
Danger
Mentions de danger
Conseils de prudence
Classification des risques
Acute Tox. 3 Oral
Code de la classe de stockage
6.1C - Combustible acute toxic Cat.3 / toxic compounds or compounds which causing chronic effects
Classe de danger pour l'eau (WGK)
WGK 3
Point d'éclair (°F)
Not applicable
Point d'éclair (°C)
Not applicable
Faites votre choix parmi les versions les plus récentes :
Certificats d'analyse (COA)
Vous ne trouvez pas la bonne version ?
Si vous avez besoin d'une version particulière, vous pouvez rechercher un certificat spécifique par le numéro de lot.
Déjà en possession de ce produit ?
Retrouvez la documentation relative aux produits que vous avez récemment achetés dans la Bibliothèque de documents.
Notre équipe de scientifiques dispose d'une expérience dans tous les secteurs de la recherche, notamment en sciences de la vie, science des matériaux, synthèse chimique, chromatographie, analyse et dans de nombreux autres domaines..
Contacter notre Service technique