O0382
Anti-Opioid δ Receptor antibody produced in rabbit
whole antiserum, lyophilized powder
About This Item
Produits recommandés
Source biologique
rabbit
Niveau de qualité
Conjugué
unconjugated
Forme d'anticorps
whole antiserum
Type de produit anticorps
primary antibodies
Clone
polyclonal
Forme
lyophilized powder
Poids mol.
antigen ~55 kDa
Espèces réactives
mouse (predicted), rat
Technique(s)
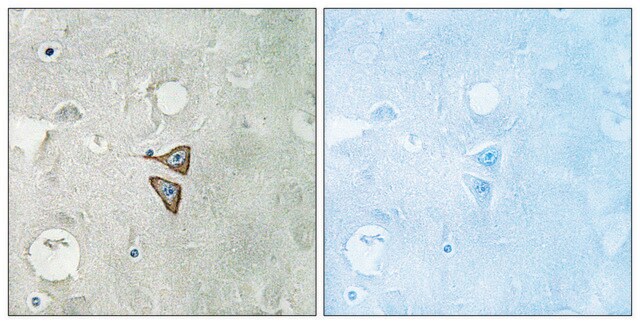
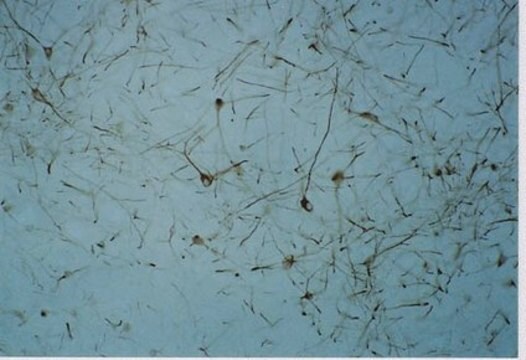
immunocytochemistry: 1:800 using PLP fixed rat brain sections
western blot: 1:800 using whole brain homogenate
Numéro d'accès UniProt
Conditions d'expédition
wet ice
Température de stockage
−20°C
Informations sur le gène
mouse ... Oprd1(18386)
rat ... Oprd1(24613)
Description générale
δ-Opioid receptors (DOR) are located postsynaptically on pallidostriatal feedback neurons. DORs also modulate nociception presynaptically in the periaqueductal gray where immunolabeling of DOR has been shown to be intracellular and often associated with large dense-core vesicles. Additionally, receptor autoradiographic investigations have localized DORs to the external plexiform layer of the olfactory bulb, the nucleus accumbens, several layers of the cerebral cortex and several nuclei of the amygdala.
References
1. Goldstein, A. Trends Pharmacol. Sci., 8, 456-459 (1987).
Spécificité
Immunogène
Clause de non-responsabilité
Vous ne trouvez pas le bon produit ?
Essayez notre Outil de sélection de produits.
Code de la classe de stockage
11 - Combustible Solids
Classe de danger pour l'eau (WGK)
WGK 3
Point d'éclair (°F)
Not applicable
Point d'éclair (°C)
Not applicable
Équipement de protection individuelle
Eyeshields, Gloves, type N95 (US)
Faites votre choix parmi les versions les plus récentes :
Déjà en possession de ce produit ?
Retrouvez la documentation relative aux produits que vous avez récemment achetés dans la Bibliothèque de documents.
Notre équipe de scientifiques dispose d'une expérience dans tous les secteurs de la recherche, notamment en sciences de la vie, science des matériaux, synthèse chimique, chromatographie, analyse et dans de nombreux autres domaines..
Contacter notre Service technique