MTOX1021
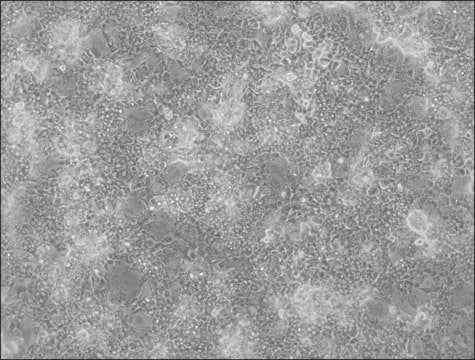
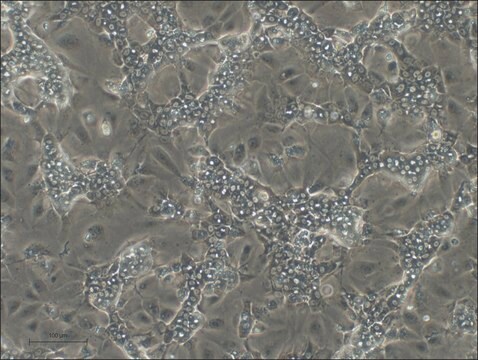
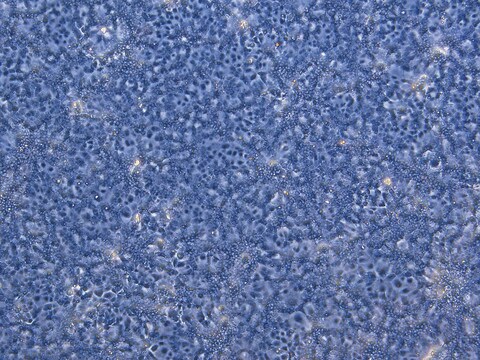
HepaRG™ Cells BCRP (-/-)
human female liver (hepatocarcinoma and hepatitis C tumor)
About This Item
Produits recommandés
Nom du produit
HepaRG™ Cells BCRP (-/-), one vial
Source biologique
human female liver (hepatocarcinoma and hepatitis C tumor)
Forme
liquid
Numéro d'accès OMIM
Température de stockage
−196°C
Informations sur le gène
human ... ABCG2(9429)
Description générale
Application
Caractéristiques et avantages
- The frame-shift mutation of ABCG2 gene was confirmed by fragment length analysis and DNA sequencing.
- Loss of functionality was confirmed by loss of transport of selective substrates in sandwich culture assay.
Qualité
Informations légales
Exhibit 2: HepaRG limited use license
Clause de non-responsabilité
Code de la classe de stockage
12 - Non Combustible Liquids
Classe de danger pour l'eau (WGK)
WGK 3
Point d'éclair (°F)
Not applicable
Point d'éclair (°C)
Not applicable
Faites votre choix parmi les versions les plus récentes :
Certificats d'analyse (COA)
It looks like we've run into a problem, but you can still download Certificates of Analysis from our Documents section.
Si vous avez besoin d'assistance, veuillez contacter Service Clients
Déjà en possession de ce produit ?
Retrouvez la documentation relative aux produits que vous avez récemment achetés dans la Bibliothèque de documents.
Articles
Oral drug delivery involves dissolution in the small intestine and absorption across the enterocyte barrier into the portal vein followed by subsequent delivery through the liver into the systemic circulation.
Notre équipe de scientifiques dispose d'une expérience dans tous les secteurs de la recherche, notamment en sciences de la vie, science des matériaux, synthèse chimique, chromatographie, analyse et dans de nombreux autres domaines..
Contacter notre Service technique