MSSAFE
Protease and Phosphatase Inhibitor Cocktail
MS-SAFE, powder, Protease inhibitors: serine, cysteine and aspartic proteases and metalloproteases. Phosphatase inhibitors: tyrosine, serine/threonine, acid and alkaline phosphatases.
Synonyme(s) :
Mass Spectrometry Safe Protease and Phosphatase Inhibitor Cocktail
Se connecterpour consulter vos tarifs contractuels et ceux de votre entreprise/organisme
About This Item
Code UNSPSC :
12352200
Nomenclature NACRES :
NA.77
Produits recommandés
Description générale
Mass spectrometry (MS) compatible protease inhibitor cocktail and phosphatase inhibitor cocktail, with broad specificity for the inhibition of:
- Serine, cysteine and aspartic proteases, and metalloproteases
- Tyrosine, serine/threonine, acid and alkaline phosphatases
Spécificité
Protease inhibitors: serine, cysteine and aspartic proteases, and metalloproteases
Phosphatase inhibitors: tyrosine, serine/threonine, acid and alkaline phosphatases
Phosphatase inhibitors: tyrosine, serine/threonine, acid and alkaline phosphatases
Application
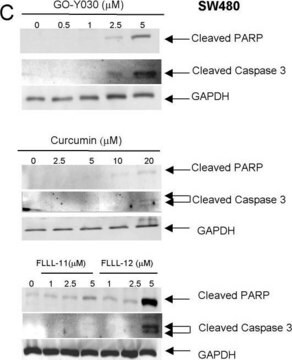
MS-SAFE Protease and Phosphatase Inhibitor has been used to measure ribosomal protein S6 kinase (S6K) activity.
Tested in mammalian cell lysates and liver tissue extracts. Designed for use in samples to be analyzed by mass spectrometry.
Caractéristiques et avantages
Comprehensive protease and phosphatase inhibitor cocktail for mass spectrometry analysis
Compatible with downstream sample processing such as His-tagged protein purification and phosphopeptide enrichment
Allows accurate measurement of protein activity and identification of phosphorylation sites.
Compatible with downstream sample processing such as His-tagged protein purification and phosphopeptide enrichment
Allows accurate measurement of protein activity and identification of phosphorylation sites.
Composants
Protease inhibitors:
- Bestatin hydrochloride
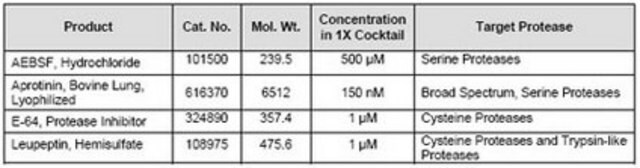
- Leupeptin
- Phosphoramidon disodium salt
- Pepstatin A
- Elastatinal
- Aprotinin
- Nafamostat mesylate
- Antipain
- Okadaic acid
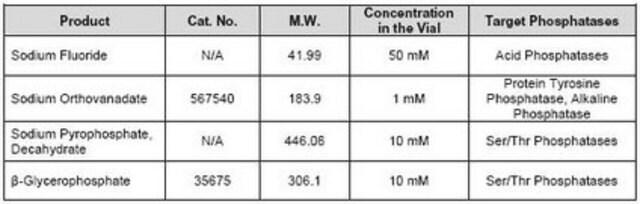
- Sodium fluoride
- Sodium orthovanadate
- Bromotetramisole oxalate
- β-lactose
- DL-leucine
Quantité
One vial makes 20 mL of 1× inhibitor cocktail working solution, using either water or extraction/lysis buffer. Alternatively, a 10× concentrated solution may be prepared by adding 2 mL of water or extraction/lysis buffer. This 10× solution may then be diluted 10-fold into extraction/lysis buffer as needed for a 1× working solution.
Forme physique
Lyophilized powder that is water-soluble
Mention d'avertissement
Warning
Mentions de danger
Conseils de prudence
Classification des risques
Acute Tox. 4 Oral - Eye Irrit. 2 - Skin Irrit. 2
Code de la classe de stockage
11 - Combustible Solids
Classe de danger pour l'eau (WGK)
WGK 3
Point d'éclair (°F)
Not applicable
Point d'éclair (°C)
Not applicable
Faites votre choix parmi les versions les plus récentes :
Déjà en possession de ce produit ?
Retrouvez la documentation relative aux produits que vous avez récemment achetés dans la Bibliothèque de documents.
Les clients ont également consulté
Mark D Schuchard et al.
BioTechniques, 39(2), 239-247 (2005-08-25)
The inclusion of protease inhibitors in serum or plasma samples has been found to significantly impact the isoform profile of selected plasma proteins as seen on 2-dimensional electrophoresis (2-DE) gels. With the addition of a protease inhibitor cocktail, several human
Targeted nanoconjugate co-delivering siRNA and tyrosine kinase inhibitor to KRAS mutant NSCLC dissociates GAB1-SHP2 post oncogene knockdown.
Srikar, R., et al.
Scientific Reports, 6, 30245-30245 (2016)
Evidence for the Role of YWHA in Mouse Oocyte Maturation
Detwiler, Ariana Claire
Thesis, 22-22 (2015)
Chandresh R Gajera et al.
Journal of neuroscience methods, 312, 73-83 (2018-11-23)
Synaptic alterations, especially presynaptic changes, are cardinal features of neurodegenerative diseases and strongly correlate with cognitive decline. We report "Mass Synaptometry" for the high-dimensional analysis of individual human synaptosomes, enriched nerve terminals from brain. This method was adapted from cytometry
Identification and characterization of PPARα ligands in the hippocampus.
Roy, A., et al.
Nature Chemical Biology, 12, 1075-1083 (2016)
Notre équipe de scientifiques dispose d'une expérience dans tous les secteurs de la recherche, notamment en sciences de la vie, science des matériaux, synthèse chimique, chromatographie, analyse et dans de nombreux autres domaines..
Contacter notre Service technique