M4414
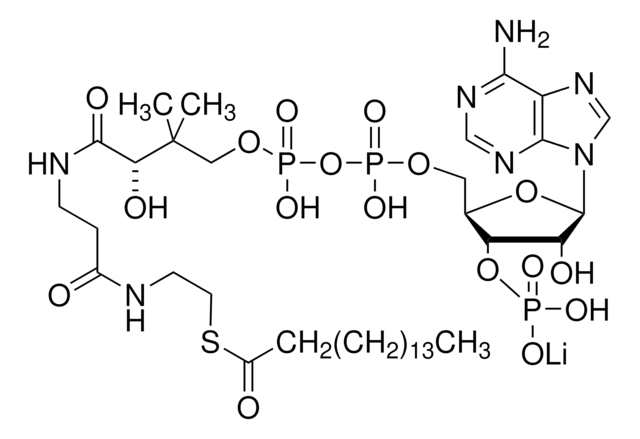
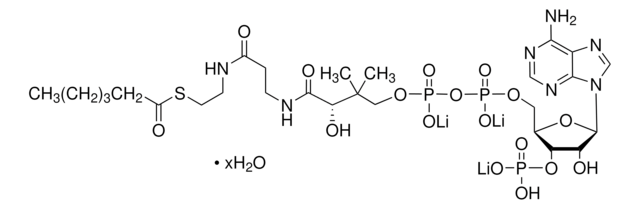
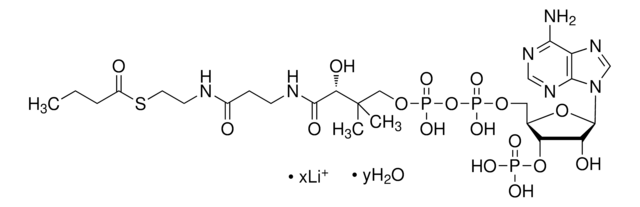
Myristoyl coenzyme A lithium salt
≥80.0% (HPLC), powder, protein myristoylation substrate
Synonyme(s) :
n-Tetradecanoyl Coenzyme A lithium salt
About This Item
Produits recommandés
product name
Myristoyl coenzyme A lithium salt, ≥80.0%
Niveau de qualité
Pureté
≥80.0%
Température de stockage
−20°C
Vous recherchez des produits similaires ? Visite Guide de comparaison des produits
Actions biochimiques/physiologiques
Caractéristiques et avantages
Substrats
Code de la classe de stockage
11 - Combustible Solids
Classe de danger pour l'eau (WGK)
WGK 3
Point d'éclair (°F)
Not applicable
Point d'éclair (°C)
Not applicable
Équipement de protection individuelle
Eyeshields, Gloves, type N95 (US)
Certificats d'analyse (COA)
Recherchez un Certificats d'analyse (COA) en saisissant le numéro de lot du produit. Les numéros de lot figurent sur l'étiquette du produit après les mots "Lot" ou "Batch".
Déjà en possession de ce produit ?
Retrouvez la documentation relative aux produits que vous avez récemment achetés dans la Bibliothèque de documents.
Les clients ont également consulté
Contenu apparenté
Cyclic nucleotides, including cyclic AMP (cAMP), cyclic GMP (cGMP) and cyclic ADP-ribose, have been extensively studied as second messengers of intracellular events initiated by activation of GPCRs. cAMP modifies cell function in all eukaryotic cells, principally through the activation of cAMP-dependent protein kinase (PKA), but also through cAMP-gated ion channels and guanine nucleotide exchange factors directly activated by cAMP.
Notre équipe de scientifiques dispose d'une expérience dans tous les secteurs de la recherche, notamment en sciences de la vie, science des matériaux, synthèse chimique, chromatographie, analyse et dans de nombreux autres domaines..
Contacter notre Service technique