G5660
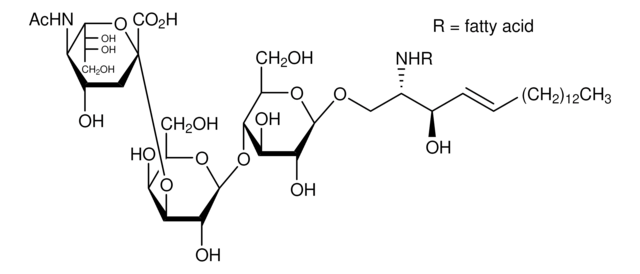
Lysoganglioside-GM1 from bovine brain
≥95%, lyophilized powder
Se connecterpour consulter vos tarifs contractuels et ceux de votre entreprise/organisme
About This Item
Produits recommandés
Catégories apparentées
Actions biochimiques/physiologiques
Suggested to be a modulator of cell growth and signal transduction. Accumulates in the nervous system of patients with GM1 gangliosidosis. Used for the preparation of GM1 derivatives containing different fatty acids, fluorescent tags, or other residues.
Autres remarques
Gangliosides are major constituents of neuronal cell membranes and endoplasmic reticulum; contain a sialated polysaccharide chain linked to ceramide through a β-glycosidic linkage; for classification of gangliosides see Svennerholm, L., et al. (eds.), Structure and Function of Gangliosides, New York, Plenum, 1980.
Code de la classe de stockage
11 - Combustible Solids
Classe de danger pour l'eau (WGK)
WGK 3
Point d'éclair (°F)
Not applicable
Point d'éclair (°C)
Not applicable
Équipement de protection individuelle
Eyeshields, Gloves, type N95 (US)
Certificats d'analyse (COA)
Recherchez un Certificats d'analyse (COA) en saisissant le numéro de lot du produit. Les numéros de lot figurent sur l'étiquette du produit après les mots "Lot" ou "Batch".
Déjà en possession de ce produit ?
Retrouvez la documentation relative aux produits que vous avez récemment achetés dans la Bibliothèque de documents.
A Kharlamov et al.
Proceedings of the National Academy of Sciences of the United States of America, 91(14), 6303-6307 (1994-07-05)
A bilateral photochemically induced thrombotic lesion of rat sensorimotor cortex (approximately 3 mm in diameter and 25 mm3 in volume) is associated with a persistent cognition (learning and memory) deficit, which was evaluated with water maze tasks. The N-dichloroacetylsphingosine derivative
Y A Hannun et al.
Science (New York, N.Y.), 235(4789), 670-674 (1987-02-06)
Lysosphingolipids potently and reversibly inhibited protein kinase C activity and binding of phorbol dibutyrate in vitro and in human platelets. As with activation of protein kinase C by phosphatidylserine and sn-1,2-diacylglycerol, inhibition was subject to surface dilution. Accordingly, inhibition in
T Kobayashi et al.
Journal of neurochemistry, 59(4), 1452-1458 (1992-10-01)
By using a sensitive method, we assayed lysocompounds of gangliosides and asialogangliosides in tissues from four patients with GM2 gangliosidosis (one with Sandhoff disease and three with Tay-Sachs disease) and from three patients with GM1 gangliosidosis [one with infantile type
Tubaro, E., et al.
Eur. J. Pharmacol. Environ. Toxicol. Pharmacol. Soc., 248, 175-175 (1993)
R T Huang et al.
FEBS letters, 281(1-2), 39-42 (1991-04-09)
Fluorescent dansyl derivatives of 3 natural sphingolipids (gangliosides, cerebroside and sphingomyelin) were shown to be readily taken up by culture cells (HeLa-, MDCK- and primary rat brain cells). A part of the incorporated fluorescent sphingolipids remained associated with the cells
Notre équipe de scientifiques dispose d'une expérience dans tous les secteurs de la recherche, notamment en sciences de la vie, science des matériaux, synthèse chimique, chromatographie, analyse et dans de nombreux autres domaines..
Contacter notre Service technique