F8512
Anti-Fibrinogen antibody produced in goat
whole antiserum
Synonyme(s) :
Anti-Fib2
Se connecterpour consulter vos tarifs contractuels et ceux de votre entreprise/organisme
About This Item
Produits recommandés
Source biologique
goat
Niveau de qualité
Conjugué
unconjugated
Forme d'anticorps
whole antiserum
Type de produit anticorps
primary antibodies
Clone
polyclonal
Contient
15 mM sodium azide
Espèces réactives
human
Technique(s)
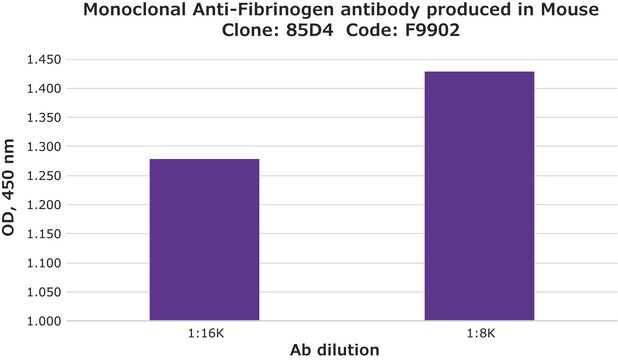
indirect ELISA: 1:10,000
quantitative precipitin assay: 2.0 mg/mL
Numéro d'accès UniProt
Température de stockage
−20°C
Modification post-traductionnelle de la cible
unmodified
Catégories apparentées
Description générale
Fibrinogen is a thrombin-coagulable soluble plasma 340 kDa glycoprotein, composed of paired sets of three subunits i.e. α, β, γ. It plays a crucial role in protecting the vascular network against the loss of blood after tissue injury. Among three subunits, β and γ subunits contain one N-glycosylation site, which is occupied by a biantennary N-glycan. It contains three pairs of disulfide-bonded chains called α, β and γ which further folded into four structural domains: the D, E, connector, and the COOH-terminal region of the Aα chain.
The fibrinogen gene cluster consists of fibrinogen α, β and γ chains. It is localized on human chromosome locus 4q31.3−4q32.1.
Spécificité
The antiserum has been determined to be immunospecific for fibrinogen by immunoelectrophoresis versus human plasma and fibrinogen.
Immunogène
human fibrinogen
Application
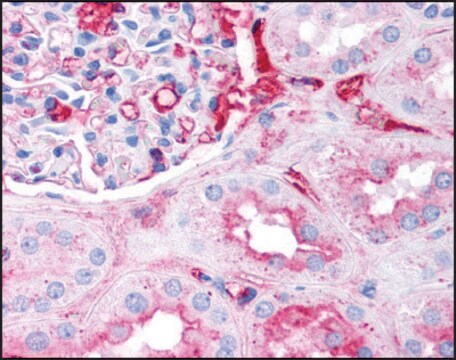
Anti-Fibrinogen antibody is suitable for immunostaining in fibrin deposition analysis of mouse livers and capturing antibodies in the sandwich ELISA. It is also suitable for indirect ELISA at a dilution of 1:10,000 and quantitative precipitin assay at 2.0mg/mL concentration.
Anti-Fibrinogen antibody produced in goat has been used in:
- western blotting detection in human colon adenocarcinoma cell line
- detecting fibrinogen in plasma
- immunoassay of human platelet free plasma (PFP)
Actions biochimiques/physiologiques
Plasmin attacks the Aα chain COOH domain to produce the heterogeneous fragment X. Multiple round of degradation ended with terminal digestion products−fragments D and E which represent the major globular domains in fibrinogen. Mutations in this gene lead to several disorders, including hypofibrinogenemia and afibrinogenemia.
Notes préparatoires
treated to remove lipoproteins
Clause de non-responsabilité
Unless otherwise stated in our catalog or other company documentation accompanying the product(s), our products are intended for research use only and are not to be used for any other purpose, which includes but is not limited to, unauthorized commercial uses, in vitro diagnostic uses, ex vivo or in vivo therapeutic uses or any type of consumption or application to humans or animals.
Vous ne trouvez pas le bon produit ?
Essayez notre Outil de sélection de produits.
Code de la classe de stockage
12 - Non Combustible Liquids
Classe de danger pour l'eau (WGK)
nwg
Point d'éclair (°F)
Not applicable
Point d'éclair (°C)
Not applicable
Faites votre choix parmi les versions les plus récentes :
Déjà en possession de ce produit ?
Retrouvez la documentation relative aux produits que vous avez récemment achetés dans la Bibliothèque de documents.
Congenital afibrinogenaemia caused by uniparental isodisomy of chromosome 4 containing a novel 15-kb deletion involving fibrinogen Aalpha-chain gene.
Spena S, et al.
European Journal of Human Genetics, 12(11), 891-891 (2004)
Integrin alphavbeta6 mediates HT29-D4 cell adhesion to MMP-processed fibrinogen in the presence of Mn2+.
Fouchier F, et al.
European Journal of Cell Biology, 86(3), 143-160 (2007)
A quantitative immunoassay for lung cancer biomarker CIZ1b in patient plasma.
Coverley D, et al.
Clinical Biochemistry, 50(6), 336-343 (2017)
Platelet adhesion and plasma protein adsorption control of collagen surfaces by He+ ion implantation.
Kurotobi K, et al.
Nucl. Instrum. Methods Phys. Res. Sect. B, 206(6), 532-537 (2003)
N E Kirschbaum et al.
The Journal of biological chemistry, 265(23), 13669-13676 (1990-08-15)
The COOH-terminal portion of the A alpha chain of human fibrinogen is highly susceptible to proteolytic degradation. This property has prevented isolation of the COOH-terminal domain of fibrinogen for the direct investigation of its functional characteristics. Human fibrinogen was degraded
Notre équipe de scientifiques dispose d'une expérience dans tous les secteurs de la recherche, notamment en sciences de la vie, science des matériaux, synthèse chimique, chromatographie, analyse et dans de nombreux autres domaines..
Contacter notre Service technique