C3788
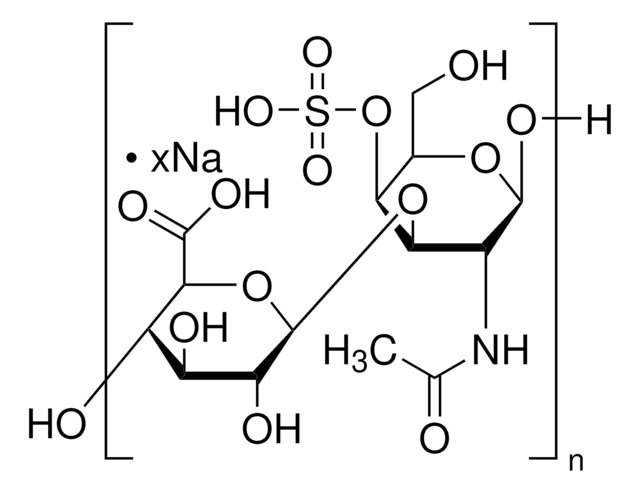
Chondroitin sulfate B sodium salt
from Porcine intestinal mucosa, ≥90%, lyophilized powder
Synonyme(s) :
β-Heparin, Dermatan sulfate sodium salt
About This Item
Produits recommandés
Source biologique
Porcine intestinal mucosa
Niveau de qualité
Essai
≥90%
Forme
lyophilized powder
Impuretés
≤20% water (Karl Fischer)
Couleur
white
Solubilité
H2O: 5 mg/mL, clear, colorless
Traces de cations
Na: 7.0-11.5%
Température de stockage
2-8°C
Vous recherchez des produits similaires ? Visite Guide de comparaison des produits
Description générale
Application
Actions biochimiques/physiologiques
Composants
Attention
Notes préparatoires
Autres remarques
Code de la classe de stockage
11 - Combustible Solids
Classe de danger pour l'eau (WGK)
WGK 3
Point d'éclair (°F)
Not applicable
Point d'éclair (°C)
Not applicable
Équipement de protection individuelle
Eyeshields, Gloves, type N95 (US)
Faites votre choix parmi les versions les plus récentes :
Déjà en possession de ce produit ?
Retrouvez la documentation relative aux produits que vous avez récemment achetés dans la Bibliothèque de documents.
Les clients ont également consulté
Articles
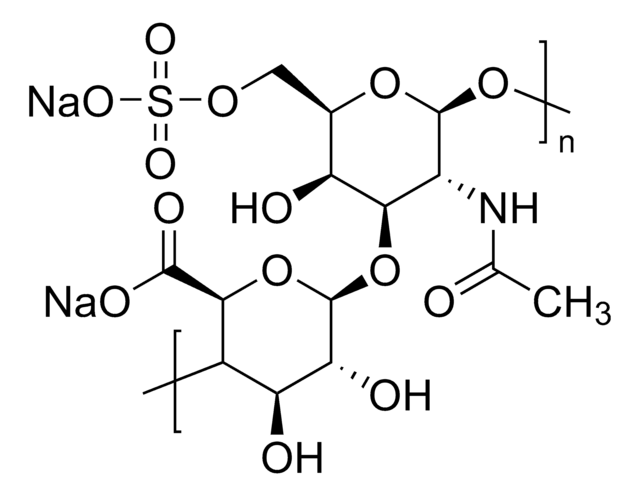
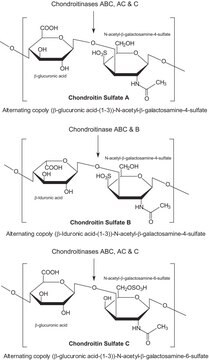
There are five identified glycosaminoglycan chains (see Figure 1): Hyaluronan is not sulfated, but the other glycosaminoglycan chains contain sulfate substituents at various positions of the chain.
Glycosaminoglycans are large linear polysaccharides constructed of repeating disaccharide units.
Notre équipe de scientifiques dispose d'une expérience dans tous les secteurs de la recherche, notamment en sciences de la vie, science des matériaux, synthèse chimique, chromatographie, analyse et dans de nombreux autres domaines..
Contacter notre Service technique