70050
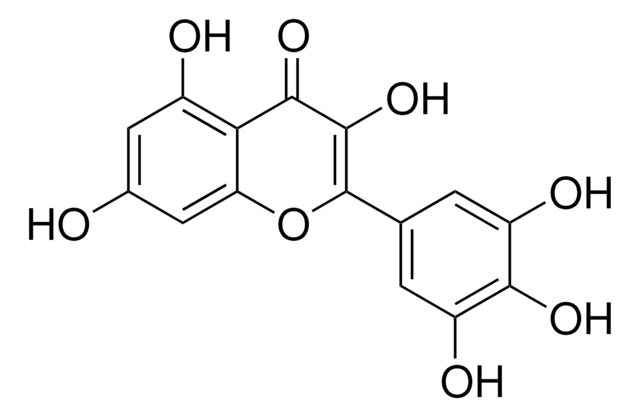
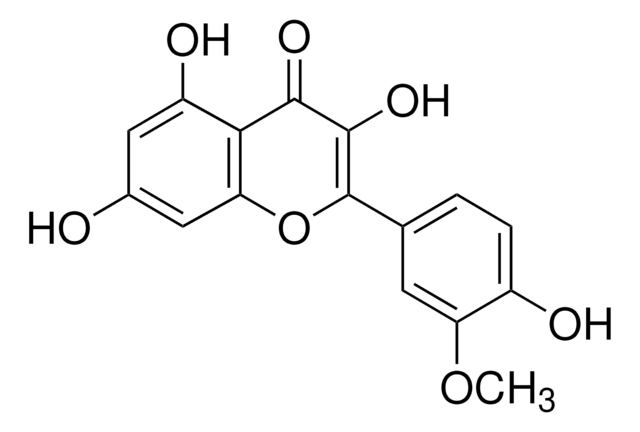
Myricetin
≥96.0% (HPLC)
Synonyme(s) :
3,3′,4′,5,5′,7-Hexahydroxyflavone, Cannabiscetin, Myricetol
About This Item
Produits recommandés
Essai
≥96.0% (HPLC)
Forme
powder
Pf
≥300 °C
>300 °C (lit.)
Solubilité
ethanol: 10 mg/mL, clear to very faintly turbid, yellow to very deep greenish-yellow
Application(s)
metabolomics
vitamins, nutraceuticals, and natural products
Chaîne SMILES
Oc1cc(O)c2C(=O)C(O)=C(Oc2c1)c3cc(O)c(O)c(O)c3
InChI
1S/C15H10O8/c16-6-3-7(17)11-10(4-6)23-15(14(22)13(11)21)5-1-8(18)12(20)9(19)2-5/h1-4,16-20,22H
Clé InChI
IKMDFBPHZNJCSN-UHFFFAOYSA-N
Informations sur le gène
human ... CYP1A2(1544)
mouse ... Hexa(15211)
rat ... Il4(287287) , Tnf(24835)
Vous recherchez des produits similaires ? Visite Guide de comparaison des produits
Description générale
Application
- to study its preventive effect as an antioxidant on noise-induced hearing loss (NIHL) in rats
- as a flavonoid compound to test antiviral activity of Bourbon virus (BRBV) and in inhibition of RNA-dependent RNA polymerase (RdRP)
- to study its effect as a treatment on biofilms of Streptococcus mutans and Candida albicans
- as a reference standard for the quantification of phenolic compounds from Juniperus species
Actions biochimiques/physiologiques
Code de la classe de stockage
11 - Combustible Solids
Classe de danger pour l'eau (WGK)
WGK 3
Point d'éclair (°F)
Not applicable
Point d'éclair (°C)
Not applicable
Équipement de protection individuelle
Eyeshields, Gloves, type N95 (US)
Faites votre choix parmi les versions les plus récentes :
Déjà en possession de ce produit ?
Retrouvez la documentation relative aux produits que vous avez récemment achetés dans la Bibliothèque de documents.
Les clients ont également consulté
Articles
Antioxidants protect biological systems from oxidative damage produced by oxygen-containing free radicals and from redoxactive transition metal ions such as iron, copper, and cadmium.
Protocoles
Coumaric acid; Quercitrin; Myricetin; Quercetin
Notre équipe de scientifiques dispose d'une expérience dans tous les secteurs de la recherche, notamment en sciences de la vie, science des matériaux, synthèse chimique, chromatographie, analyse et dans de nombreux autres domaines..
Contacter notre Service technique