17938
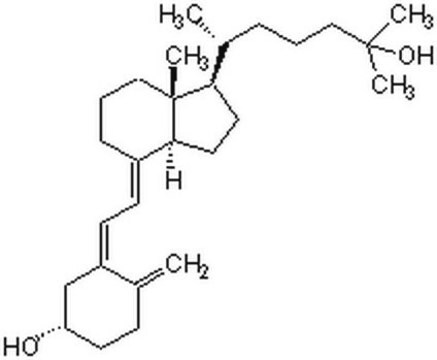
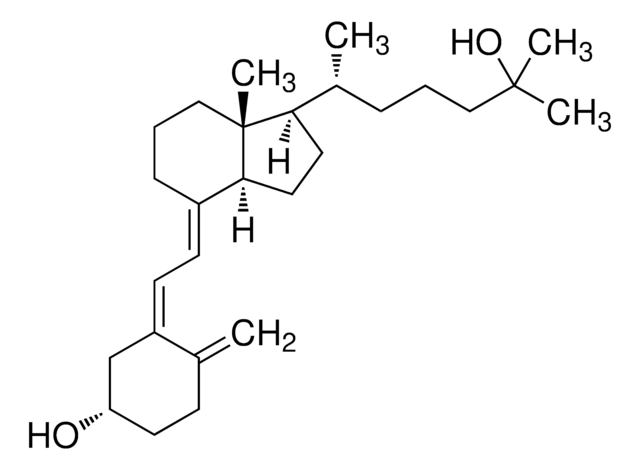
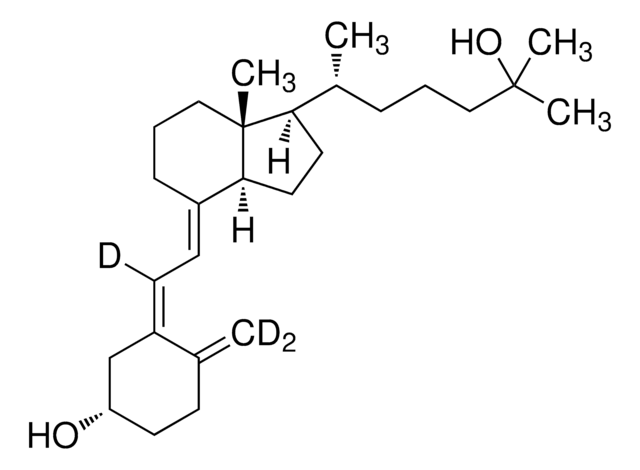
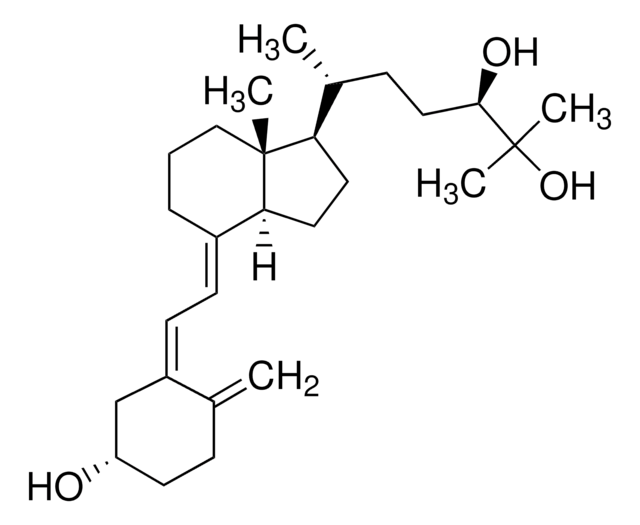
25-Hydroxyvitamin D3 monohydrate
≥99.0% (HPLC)
Synonyme(s) :
25-Hydroxycholecalciferol
About This Item
Produits recommandés
Source biologique
synthetic
Niveau de qualité
Pureté
≥99.0% (HPLC)
Forme
powder or crystals
Couleur
white to off-white
Conditions d'expédition
dry ice
Température de stockage
−20°C
Chaîne SMILES
[H][C@@]1(CC[C@@]2([H])C(\CCC[C@]12C)=C\C=C3\C[C@@H](O)CCC3=C)[C@H](C)CCCC(C)(C)O
InChI
1S/C27H44O2/c1-19-10-13-23(28)18-22(19)12-11-21-9-7-17-27(5)24(14-15-25(21)27)20(2)8-6-16-26(3,4)29/h11-12,20,23-25,28-29H,1,6-10,13-18H2,2-5H3/b21-11+,22-12-/t20-,23+,24-,25+,27-/m1/s1
Clé InChI
JWUBBDSIWDLEOM-DTOXIADCSA-N
Informations sur le gène
human ... VDR(7421)
Vous recherchez des produits similaires ? Visite Guide de comparaison des produits
Actions biochimiques/physiologiques
Conditionnement
Produit(s) apparenté(s)
Mention d'avertissement
Danger
Mentions de danger
Conseils de prudence
Classification des risques
Acute Tox. 2 Inhalation - Acute Tox. 3 Dermal - Acute Tox. 3 Oral - STOT RE 1 Oral
Code de la classe de stockage
6.1A - Combustible acute toxic Cat. 1 and 2 / very toxic hazardous materials
Classe de danger pour l'eau (WGK)
WGK 3
Point d'éclair (°F)
Not applicable
Point d'éclair (°C)
Not applicable
Équipement de protection individuelle
Eyeshields, Faceshields, Gloves, type P3 (EN 143) respirator cartridges
Certificats d'analyse (COA)
Recherchez un Certificats d'analyse (COA) en saisissant le numéro de lot du produit. Les numéros de lot figurent sur l'étiquette du produit après les mots "Lot" ou "Batch".
Déjà en possession de ce produit ?
Retrouvez la documentation relative aux produits que vous avez récemment achetés dans la Bibliothèque de documents.
Les clients ont également consulté
Articles
Vitamin D2 (ergocalciferol) is naturally synthesized from ergosterol by invertebrates, fungi, and plants in response to ultraviolet B irradiation, while vitamin D3 synthesis (cholecalciferol) is uniquely initiated in the skin of vertebrates. During sun exposure, ultraviolet B photons are absorbed by 7-dehydrocholesterol, which is found within the plasma membranes of epidermal and dermal skin layers. This reaction yields an unstable derivative of 7-dehydrocholesterol, named precholecalcitrol, which rapidly rearranges to vitamin D3. Vitamin D binding protein (DBP) is a carrier protein responsible for drawing vitamin D3 from the plasma membrane into the dermal capillaries within the extracellular space.
Protocoles
While quantitative analysis was performed for Vitamins D2 and D3, the samples were scanned for the presence of the 25-hydroxy metabolites.
Notre équipe de scientifiques dispose d'une expérience dans tous les secteurs de la recherche, notamment en sciences de la vie, science des matériaux, synthèse chimique, chromatographie, analyse et dans de nombreux autres domaines..
Contacter notre Service technique