383120
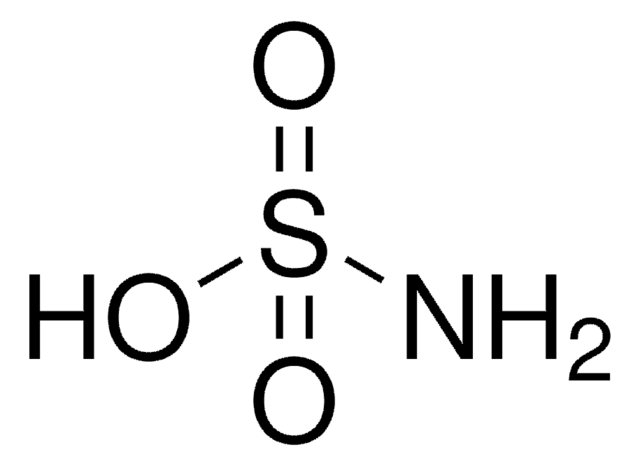
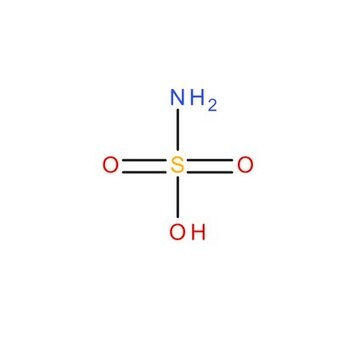
Sulfamic acid
ACS reagent, 99.3%
Synonyme(s) :
Amidosulfonic acid
About This Item
Produits recommandés
Qualité
ACS reagent
Essai
99.3%
99.3-100.3% dry basis (ACS specification)
Forme
crystals
Technique(s)
titration: suitable
Impuretés
≤0.01% insolubles
Résidus de calcination
≤0.01%
Pf
215-225 °C (dec.) (lit.)
Solubilité
water: 213 g/L at 20 °C
Densité
2.151 g/cm3 at 25 °C
Traces d'anions
chloride (Cl-): ≤0.001%
sulfate (SO42-): ≤0.05%
Traces de cations
Fe: ≤5 ppm
heavy metals (as Pb): ≤0.001%
Chaîne SMILES
NS(O)(=O)=O
InChI
1S/H3NO3S/c1-5(2,3)4/h(H3,1,2,3,4)
Clé InChI
IIACRCGMVDHOTQ-UHFFFAOYSA-N
Vous recherchez des produits similaires ? Visite Guide de comparaison des produits
Catégories apparentées
Description générale
Application
- As catalyst in the synthesis of aryl-14H-dibenzo[a.j]xanthenes.
- As green catalyst for the preparation of amide from ketoxime.
- As ammonia equivalent in the regioselective synthesis of primary allylic amines, via allylic substitution reactions.
- Synthesis of polysubstituted quinolones.
- As a titrant in the determination of the burette injection volume and chemical calibration factor.
- To neutralize excess nitrous acid in the colorimetric paracetamol assay by modified Glynn and Kendal colorimetric method.
- To prevent endogenous mercury (Hg) loss during the urine Hg measurement by inductively coupled plasma mass spectrometry (ICP-MS) method.
- As an acid catalyst and a hypochlorite scavenger in the chlorite oxidation of dialdehyde cellulose (DAC).
- As a heterogeneous catalyst in the synthesis of polyhydroquinoline derivatives by Hantzsch condensation reaction.
- As catalyst in the degradation of bamboo fiber to 5-hydroxymethylfurfural (HMF).
- As an acid reagent in the determination of silicates in water samples based on centrifugal microfluidics.
- As catalyst in the synthesis of deazaoxaflavin at room temperature.
Mention d'avertissement
Warning
Mentions de danger
Conseils de prudence
Classification des risques
Aquatic Chronic 3 - Eye Irrit. 2 - Skin Irrit. 2
Code de la classe de stockage
8B - Non-combustible corrosive hazardous materials
Classe de danger pour l'eau (WGK)
WGK 1
Point d'éclair (°F)
Not applicable
Point d'éclair (°C)
Not applicable
Faites votre choix parmi les versions les plus récentes :
Déjà en possession de ce produit ?
Retrouvez la documentation relative aux produits que vous avez récemment achetés dans la Bibliothèque de documents.
Les clients ont également consulté
Notre équipe de scientifiques dispose d'une expérience dans tous les secteurs de la recherche, notamment en sciences de la vie, science des matériaux, synthèse chimique, chromatographie, analyse et dans de nombreux autres domaines..
Contacter notre Service technique