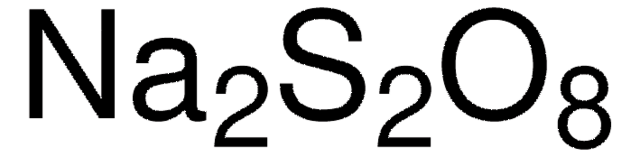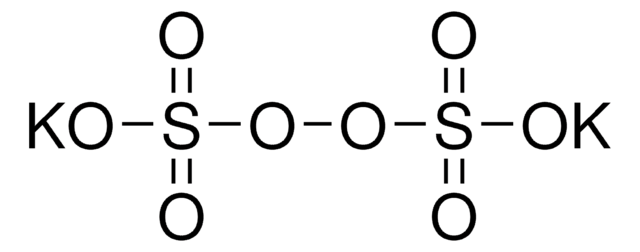S6172
Sodium persulfate
BioXtra, ≥99%
Synonyme(s) :
Sodium peroxodisulfate
About This Item
Produits recommandés
Gamme de produits
BioXtra
Niveau de qualité
Essai
≥99%
Pertinence de la réaction
reagent type: oxidant
Impuretés
<0.0005% Phosphorus (P)
<0.1% Insoluble matter
Solubilité
H2O: 1 M at 20 °C, clear, colorless
Traces d'anions
chloride (Cl-): <0.05%
Traces de cations
Al: <0.0005%
Ca: <0.005%
Cu: <0.0005%
Fe: <0.0005%
K: <0.02%
Mg: <0.001%
Pb: <0.001%
Zn: <0.0005%
Chaîne SMILES
[Na+].[Na+].[O-]S(=O)(=O)OOS([O-])(=O)=O
InChI
1S/2Na.H2O8S2/c;;1-9(2,3)7-8-10(4,5)6/h;;(H,1,2,3)(H,4,5,6)/q2*+1;/p-2
Clé InChI
CHQMHPLRPQMAMX-UHFFFAOYSA-L
Vous recherchez des produits similaires ? Visite Guide de comparaison des produits
Application
<li><strong>Electrochemical treatment of organic pollutants in landfill leachate using a three-dimensional electrode system.</strong>: This study explores the electrochemical treatment of landfill leachate using a three-dimensional electrode system. Sodium persulfate is used as an oxidizing agent to degrade organic pollutants effectively, providing a potential method for waste management and environmental protection (Yu et al., 2020).</li>
<li><strong>The Box-Benkhen experimental design for the optimization of the electrocatalytic treatment of wastewaters with high concentrations of phenol and organic matter.</strong>: This paper discusses the optimization of electrocatalytic treatment processes for wastewater containing high levels of phenol and organic matter using sodium persulfate. The study provides valuable insights for improving wastewater treatment efficiency (GilPavas et al., 2009).</li>
<li><strong>Reaction of pectin and glycidyl methacrylate and ulterior formation of free films by reticulation.</strong>: This research involves the chemical modification of pectin with glycidyl methacrylate followed by cross-linking using sodium persulfate, leading to the formation of free-standing films. These films have potential applications in pharmaceuticals and food packaging (Maior et al., 2008).</li>
</ul>
Vous ne trouvez pas le bon produit ?
Essayez notre Outil de sélection de produits.
Mention d'avertissement
Danger
Mentions de danger
Conseils de prudence
Classification des risques
Acute Tox. 4 Oral - Ox. Sol. 3 - Resp. Sens. 1 - Skin Irrit. 2 - Skin Sens. 1 - STOT SE 3
Organes cibles
Respiratory system
Code de la classe de stockage
5.1B - Oxidizing hazardous materials
Classe de danger pour l'eau (WGK)
WGK 1
Point d'éclair (°F)
Not applicable
Point d'éclair (°C)
Not applicable
Équipement de protection individuelle
Eyeshields, Faceshields, Gloves, type P3 (EN 143) respirator cartridges
Faites votre choix parmi les versions les plus récentes :
Déjà en possession de ce produit ?
Retrouvez la documentation relative aux produits que vous avez récemment achetés dans la Bibliothèque de documents.
Les clients ont également consulté
Notre équipe de scientifiques dispose d'une expérience dans tous les secteurs de la recherche, notamment en sciences de la vie, science des matériaux, synthèse chimique, chromatographie, analyse et dans de nombreux autres domaines..
Contacter notre Service technique