K1637
Kanamycine sulfate from Streptomyces kanamyceticus
meets USP testing specifications, powder
Synonyme(s) :
Kanamycine sulfate salt, Kanamycine A
About This Item
Produits recommandés
Source biologique
Streptomyces kanamyceticus
Niveau de qualité
Agence
USP/NF
meets USP testing specifications
Forme
powder
Puissance
≥750 μg per mg
Solubilité
H2O: 10-50 mg/mL (As a stock solution. Stock solutions should be stored at 2-8°C. Stable at 37°C for 5 days.)
Spectre d'activité de l'antibiotique
Gram-negative bacteria
Gram-positive bacteria
mycobacteria
mycoplasma
Application(s)
pharmaceutical (small molecule)
Mode d’action
protein synthesis | interferes
Chaîne SMILES
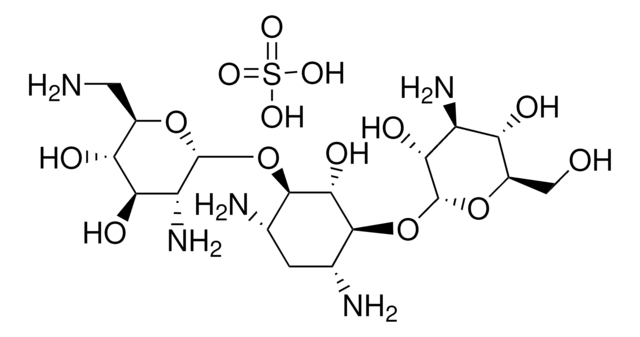
OS(O)(=O)=O.NC[C@H]1O[C@H](O[C@@H]2[C@@H](N)C[C@@H](N)[C@H](O[C@H]3O[C@H](CO)[C@@H](O)[C@H](N)[C@H]3O)[C@H]2O)[C@H](O)[C@@H](O)[C@@H]1O
InChI
1S/C18H36N4O11.H2O4S/c19-2-6-10(25)12(27)13(28)18(30-6)33-16-5(21)1-4(20)15(14(16)29)32-17-11(26)8(22)9(24)7(3-23)31-17;1-5(2,3)4/h4-18,23-29H,1-3,19-22H2;(H2,1,2,3,4)/t4-,5+,6-,7-,8+,9-,10-,11-,12+,13-,14-,15+,16-,17-,18-;/m1./s1
Clé InChI
OOYGSFOGFJDDHP-KMCOLRRFSA-N
Vous recherchez des produits similaires ? Visite Guide de comparaison des produits
Description générale
Application
Actions biochimiques/physiologiques
Mode de résistance : les enzymes modifiant les aminoglycosides (dont l'acétyltransférase, la phosphotransférase et la nucléotidyltransférase) peuvent modifier cet antibiotique, l'empêchant alors d'interagir avec les ribosomes.
Spectre antimicrobien : Le sulfate de kanamycine est efficace sur les bactéries à Gram négatif, les bactéries à Gram positif et les mycoplasmes.
Caractéristiques et avantages
- High quality antibiotic suitable for mulitple research applications
- meets USP testing specifications
Notes préparatoires
Solutions are stable at 37°C for approximately 5 days. Aqueous stock solutions can be stored at 2-8°C for long term storage.
Stockage et stabilité
Autres remarques
Produit comparable
Mention d'avertissement
Danger
Mentions de danger
Conseils de prudence
Classification des risques
Repr. 1B
Code de la classe de stockage
6.1D - Non-combustible acute toxic Cat.3 / toxic hazardous materials or hazardous materials causing chronic effects
Classe de danger pour l'eau (WGK)
WGK 2
Équipement de protection individuelle
Eyeshields, Gloves, type N95 (US)
Faites votre choix parmi les versions les plus récentes :
Déjà en possession de ce produit ?
Retrouvez la documentation relative aux produits que vous avez récemment achetés dans la Bibliothèque de documents.
Les clients ont également consulté
Notre équipe de scientifiques dispose d'une expérience dans tous les secteurs de la recherche, notamment en sciences de la vie, science des matériaux, synthèse chimique, chromatographie, analyse et dans de nombreux autres domaines..
Contacter notre Service technique