919764
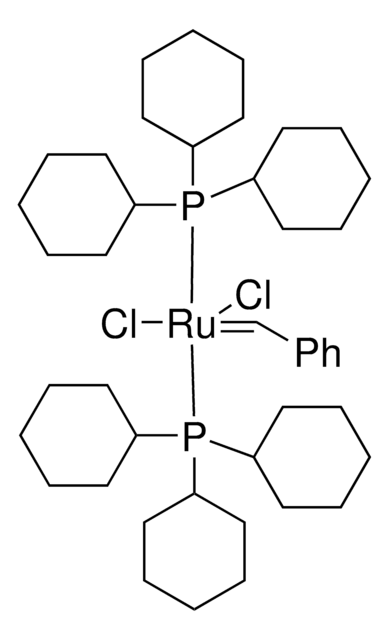
Grubbs Catalyst® M204 ChemBeads
Synonyme(s) :
(1,3-Bis(2,4,6-trimethylphenyl)-2-imidazolidinylidene)dichloro(phenylmethylene)(tricyclohexylphosphine)ruthenium, Benzylidene[1,3-bis(2,4,6-trimethylphenyl)-2-imidazolidinylidene]dichloro(tricyclohexylphosphine)ruthenium, Dichloro[1,3-bis(2,4,6-trimethylphenyl)-2-imidazolidinylidene](benzylidene)(tricyclohexylphosphine)ruthenium(II), Grubbs Catalyst® 2nd Generation, Grubbs Catalyst® M2a (C848)
About This Item
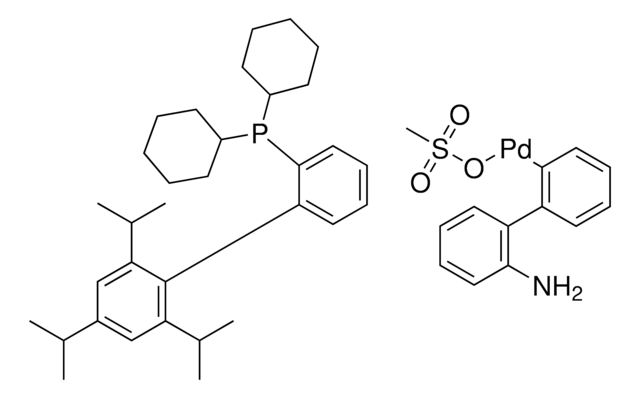
Produits recommandés
Forme
solid
Niveau de qualité
Composition
~ 4 wt.% loading of catalyst
Pertinence de la réaction
reagent type: catalyst
core: ruthenium
reaction type: Ring-Opening Polymerization
Température de stockage
2-8°C
Chaîne SMILES
CC1=CC(C)=CC(C)=C1N(CCN2C3=C(C=C(C=C3C)C)C)[C-2]2=[Ru+6](P(C4CCCCC4)(C5CCCCC5)C6CCCCC6)([Cl-])([Cl-])=[C-2]C7=CC=CC=C7
InChI
1S/C21H26N2.C18H33P.C7H6.2ClH.Ru/c1-14-9-16(3)20(17(4)10-14)22-7-8-23(13-22)21-18(5)11-15(2)12-19(21)6;1-4-10-16(11-5-1)19(17-12-6-2-7-13-17)18-14-8-3-9-15-18;1-7-5-3-2-4-6-7;;;/h9-12H,7-8H2,1-6H3;16-18H,1-15H2;1-6H;2*1H;/q;;;;;+2/p-2
Clé InChI
FCDPQMAOJARMTG-UHFFFAOYSA-L
Catégories apparentées
Application
It can also be used as a catalyst:
To synthesize coumarins from phenolic compounds via RCM.
To cleave secondary (E)-allyl vic-diols to aldehydes.
ChemBeads are chemical coated glass beads. ChemBeads offer improved flowability and chemical uniformity perfect for automated solid dispensing and high-throughput experimentation. The method of creating ChemBeads uses no other chemicals or surfactants allowing the user to accurately dispense sub-milligram amounts of chemical.
Learn more about ChemBeads products
For larger scale uses, product is also available in powdered form (569747)
Autres remarques
Grubbs Catalyst Technology for Olefin Metathesis by Aldrich
High-Throughput Reaction Screening with Nanomoles of Solid Reagents Coated on Glass Beads
Versatile Methods to Dispense Sub-Milligram Quantities of Solids using Chemical Coated Beads for High-Throughput Experimentation
ChemBead Enabled High-Throughput Cross-Electrophile Coupling Reveals a New Complementary Ligand
Informations légales
Produit(s) apparenté(s)
Mention d'avertissement
Danger
Mentions de danger
Conseils de prudence
Classification des risques
Carc. 1B Inhalation - Flam. Sol. 2
Code de la classe de stockage
4.1B - Flammable solid hazardous materials
Classe de danger pour l'eau (WGK)
WGK 3
Point d'éclair (°F)
Not applicable
Point d'éclair (°C)
Not applicable
Faites votre choix parmi les versions les plus récentes :
Certificats d'analyse (COA)
Vous ne trouvez pas la bonne version ?
Si vous avez besoin d'une version particulière, vous pouvez rechercher un certificat spécifique par le numéro de lot.
Déjà en possession de ce produit ?
Retrouvez la documentation relative aux produits que vous avez récemment achetés dans la Bibliothèque de documents.
Notre équipe de scientifiques dispose d'une expérience dans tous les secteurs de la recherche, notamment en sciences de la vie, science des matériaux, synthèse chimique, chromatographie, analyse et dans de nombreux autres domaines..
Contacter notre Service technique




![[1,3-Bis(2,4,6-trimethylphenyl)-2-imidazolidinylidene]dichloro(benzylidene)bis(3-bromopyridine)ruthenium(II)](/deepweb/assets/sigmaaldrich/product/structures/261/898/e64eea4e-5a09-4c7d-b400-c43b3517de2a/640/e64eea4e-5a09-4c7d-b400-c43b3517de2a.png)