414247
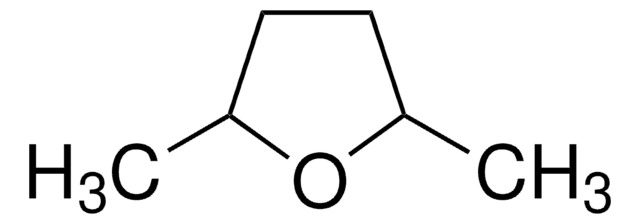
2-Methyltetrahydrofuran
BioRenewable, anhydrous, ≥99.0%, contains 250 ppm BHT as stabilizer
Synonyme(s) :
Tétrahydro-2-méthylfurane
About This Item
Produits recommandés
Qualité
anhydrous
Niveau de qualité
Pureté
≥99.0%
Forme
liquid
Contient
250 ppm BHT as stabilizer
Limite d'explosivité
0.34-6.3 %
Caractéristiques du produit alternatif plus écologique
Safer Solvents and Auxiliaries
Use of Renewable Feedstocks
Learn more about the Principles of Green Chemistry.
sustainability
Greener Alternative Product
Impuretés
<0.002% water
<0.005% water (100 mL pkg)
Résidus d'évap.
<0.0003%
Indice de réfraction
n20/D 1.406 (lit.)
Point d'ébullition
78-80 °C (lit.)
Pf
-136 °C
Densité
0.86 g/mL at 25 °C (lit.)
Chaîne SMILES
CC1CCCO1
InChI
1S/C5H10O/c1-5-3-2-4-6-5/h5H,2-4H2,1H3
Clé InChI
JWUJQDFVADABEY-UHFFFAOYSA-N
Vous recherchez des produits similaires ? Visite Guide de comparaison des produits
Description générale
2-Methyltetrahydrofuran (2-MTHF), a 2-methyl substituted tetrahdrofuran, is a biomass derived solvent. It is a potential greener solvent alternative for organic synthesis. It shows resistance to reduction by lithium making it a promising candidate as electrolytes in lithium batteries. Its polarity and Lewis base strength is intermediate between tetrahydrofuran (THF) and diethyl ether. The ring opening reaction of 2-MTHF has been studied using acid chloride and iodide to form secondary chlorides and primary iodides respectively. On long term storage, tetrahydrofuran forms organic peroxides. This process can be suppressed by adding butylated hydroxytoluene (BHT) as a stabilizer. BHT removes the free radicals required for the peroxide formation.
Application
It may be used as an alternative solvent to:
- DMSO (dimethyl sulfoxide) or MTBE (methyl tertiary butyl ether) in the C-C bond forming reactions catalyzed by lyase enzyme.
- THF in the reaction between Grignard reagents and carbonyl compounds.
- Methylene chloride in some biphase reactions.
Organic Solar Cells
2-Methyltetrahydrofuran (2-MeTHF): A Biomass-Derived Solvent with Broad Application in Organic Chemistry
Caractéristiques et avantages
Conditionnement
Autres remarques
Informations légales
Mention d'avertissement
Danger
Mentions de danger
Conseils de prudence
Classification des risques
Acute Tox. 4 Oral - Eye Dam. 1 - Flam. Liq. 2 - Skin Irrit. 2
Risques supp
Code de la classe de stockage
3 - Flammable liquids
Classe de danger pour l'eau (WGK)
WGK 2
Point d'éclair (°F)
14.0 °F - closed cup
Point d'éclair (°C)
-10.0 °C - closed cup
Équipement de protection individuelle
Eyeshields, Faceshields, Gloves, type ABEK (EN14387) respirator filter
Choose from one of the most recent versions:
Déjà en possession de ce produit ?
Retrouvez la documentation relative aux produits que vous avez récemment achetés dans la Bibliothèque de documents.
Les clients ont également consulté
Notre équipe de scientifiques dispose d'une expérience dans tous les secteurs de la recherche, notamment en sciences de la vie, science des matériaux, synthèse chimique, chromatographie, analyse et dans de nombreux autres domaines..
Contacter notre Service technique