33047
Acide benzoique
puriss. p.a., ACS reagent, reag. Ph. Eur., ≥99.9% (alkalimetric)
Synonyme(s) :
Benzenecarboxylic acid, Carboxybenzene
About This Item
Produits recommandés
Qualité
ACS reagent
puriss. p.a.
Niveau de qualité
Agence
reag. Ph. Eur.
Densité de vapeur
4.21 (vs air)
Pression de vapeur
10 mmHg ( 132 °C)
Pureté
≥99.9% (alkalimetric)
Forme
crystalline
Température d'inflammation spontanée
1061 °F
Impuretés
readily oxidisable substances, in accordance
≤0.002% S-compounds (as S)
≤0.005% halogen compounds (as Cl)
≤0.005% insoluble in methanol
Résidus de calcination
≤0.005% (as SO4)
Point d'ébullition
249 °C (lit.)
Pf
121-125 °C (lit.)
Solubilité
water: soluble (2.9 g/l at 25 °C)
Traces d'anions
sulfate (SO42-): ≤20 mg/kg
Traces de cations
Cu: ≤5 mg/kg
Fe: ≤5 mg/kg
Pb: ≤2 mg/kg
Zn: ≤5 mg/kg
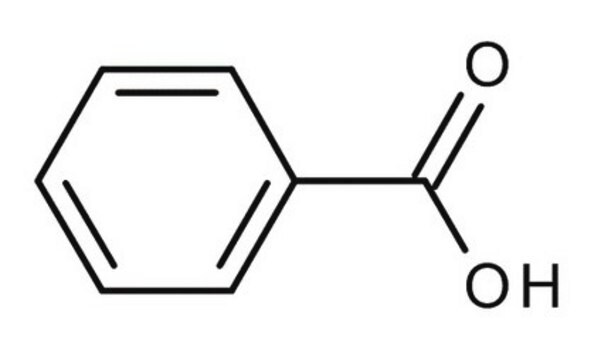
Chaîne SMILES
OC(=O)c1ccccc1
InChI
1S/C7H6O2/c8-7(9)6-4-2-1-3-5-6/h1-5H,(H,8,9)
Clé InChI
WPYMKLBDIGXBTP-UHFFFAOYSA-N
Vous recherchez des produits similaires ? Visite Guide de comparaison des produits
Catégories apparentées
Description générale
Application
- paints
- pigments
- varnish
- wetting agents
- aroma compounds
- benzoyl chloride
- benzotrichloride
Mention d'avertissement
Danger
Mentions de danger
Conseils de prudence
Classification des risques
Eye Dam. 1 - Skin Irrit. 2 - STOT RE 1 Inhalation
Organes cibles
Lungs
Code de la classe de stockage
6.1C - Combustible, acute toxic Cat.3 / toxic compounds or compounds which causing chronic effects
Classe de danger pour l'eau (WGK)
WGK 1
Point d'éclair (°F)
Not applicable
Point d'éclair (°C)
Not applicable
Équipement de protection individuelle
dust mask type N95 (US), Eyeshields, Faceshields, Gloves
Choose from one of the most recent versions:
Déjà en possession de ce produit ?
Retrouvez la documentation relative aux produits que vous avez récemment achetés dans la Bibliothèque de documents.
Les clients ont également consulté
Notre équipe de scientifiques dispose d'une expérience dans tous les secteurs de la recherche, notamment en sciences de la vie, science des matériaux, synthèse chimique, chromatographie, analyse et dans de nombreux autres domaines..
Contacter notre Service technique