08198
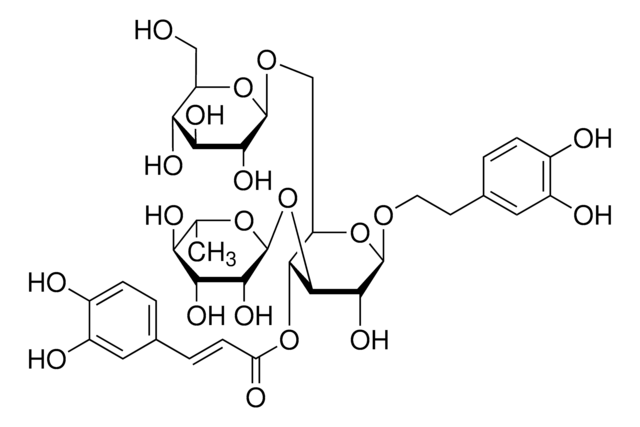
Eleutheroside E
analytical standard
Synonyme(s) :
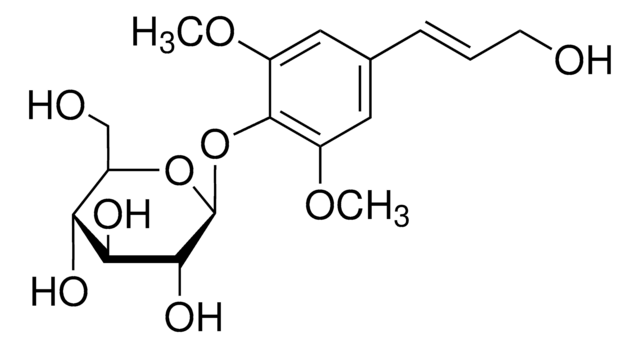
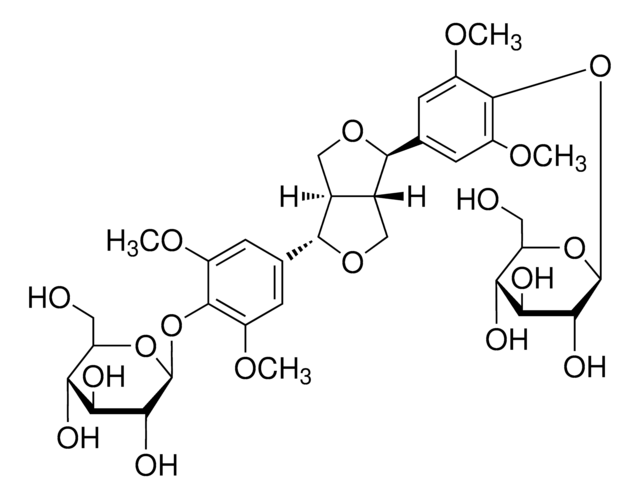
[(1R,3aR,4S,6aS)-Tetrahydro-1H,3H-furo[3,4-c]furan-1,4-diyl]bis(2,6-dimethoxy-4,1-phenylene) bis-β-D-glucopyranoside
About This Item
Produits recommandés
Qualité
analytical standard
Niveau de qualité
Pureté
≥98.0% (HPLC)
Durée de conservation
limited shelf life, expiry date on the label
Application(s)
food and beverages
Format
neat
Température de stockage
2-8°C
Chaîne SMILES
COc1cc(cc(OC)c1O[C@@H]2O[C@H](CO)[C@@H](O)[C@H](O)[C@H]2O)[C@H]3OC[C@@H]4[C@@H]3CO[C@H]4c5cc(OC)c(O[C@@H]6O[C@H](CO)[C@@H](O)[C@H](O)[C@H]6O)c(OC)c5
InChI
1S/C34H46O18/c1-43-17-5-13(6-18(44-2)31(17)51-33-27(41)25(39)23(37)21(9-35)49-33)29-15-11-48-30(16(15)12-47-29)14-7-19(45-3)32(20(8-14)46-4)52-34-28(42)26(40)24(38)22(10-36)50-34/h5-8,15-16,21-30,33-42H,9-12H2,1-4H3/t15?,16?,21-,22+,23-,24+,25+,26-,27-,28+,29?,30?,33+,34-
Clé InChI
FFDULTAFAQRACT-RGFZIUCCSA-N
Vous recherchez des produits similaires ? Visite Guide de comparaison des produits
Description générale
Application
- Rat plasma and tissue by solid-phase extraction (SPE) followed by high-performance liquid chromatography (HPLC) and photodiode array detection (PDA).
- Acanthopanax senticosus by ionic liquids-ultrasound assisted extraction (ILUAE) followed by HPLC with ultraviolet (UV) detection.
- Eleutherococcus senticosus Maxim. by rapid resolution liquid chromatography (RRLC) equipped with multi-wavelength UV detector.
- Acanthopanax giraldii Harms by HPLC with diode array detector (DAD).
Conditionnement
Code de la classe de stockage
11 - Combustible Solids
Classe de danger pour l'eau (WGK)
WGK 3
Point d'éclair (°F)
Not applicable
Point d'éclair (°C)
Not applicable
Faites votre choix parmi les versions les plus récentes :
Déjà en possession de ce produit ?
Retrouvez la documentation relative aux produits que vous avez récemment achetés dans la Bibliothèque de documents.
Notre équipe de scientifiques dispose d'une expérience dans tous les secteurs de la recherche, notamment en sciences de la vie, science des matériaux, synthèse chimique, chromatographie, analyse et dans de nombreux autres domaines..
Contacter notre Service technique