SCR526M
Alzheimer′s In A Dish™ APPSL-GFP Lentivirus
Synonyme(s) :
APPSL-GFP , ReNcell 3D Alzheimers Model, ADID 3D Neural Cell Model
About This Item
Produits recommandés
Espèces réactives
human
Niveau de qualité
Technique(s)
cell culture | mammalian: suitable
cell culture | stem cell: suitable
cell differentiation: suitable
Description générale
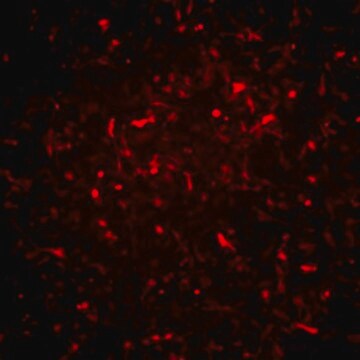
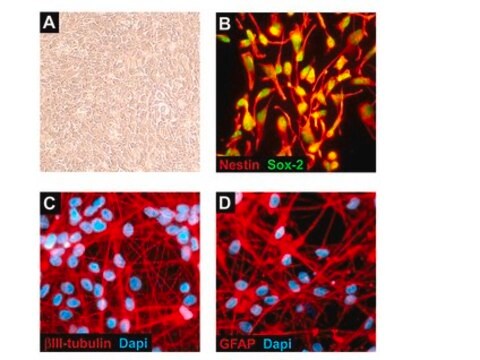
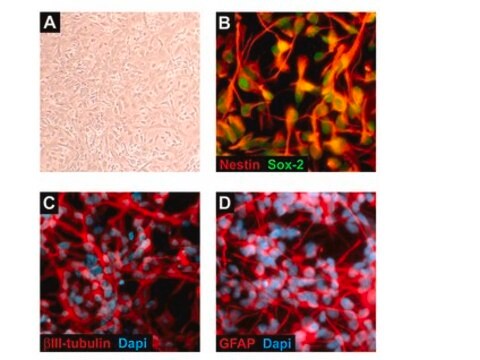
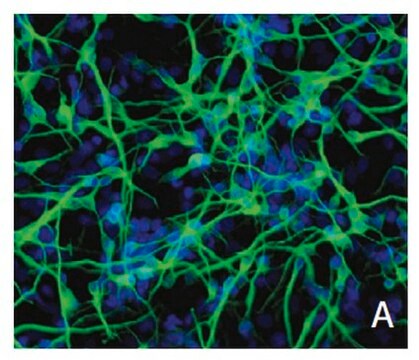
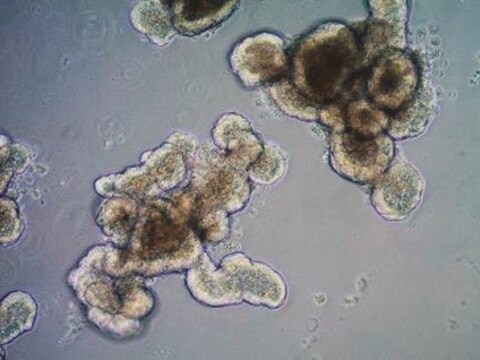
Recently a 3D model using genetically engineered human neural stem cells that overexpress FAD mutations was reported to recapitulate the two pathological hallmarks of AD: b-amyloid plaques and neurofibrillary tangles. Lentiviruses expressing FAD mutations in human APP with both the K670N/M671L (Swedish) and V717I (London) mutations (APPSL) and/or PSEN1 with the E9 mutation (PSEN1(E9)) and APPSL/PSEN1(E9) along with fluorescent proteins as reporters for viral infection (see below), were used to transfect ReNcell VM human neural stem cells. FACS was utilized to enrich for cells with the highest transgene expressions followed by encapsulation of the sorted cells in a 3D Matrigel culture system. After approximately 6 weeks of differentiation, aggregation of AB was observed. Robust accumulation of phosphorylated tau along neurofibrillary tangles was readily detectable after 10-14 weeks.
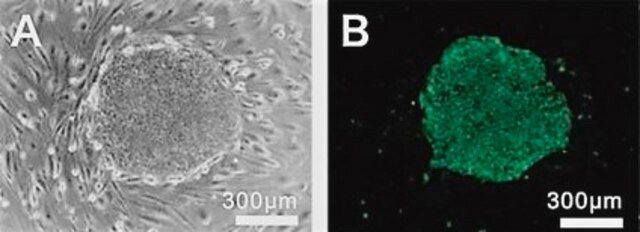
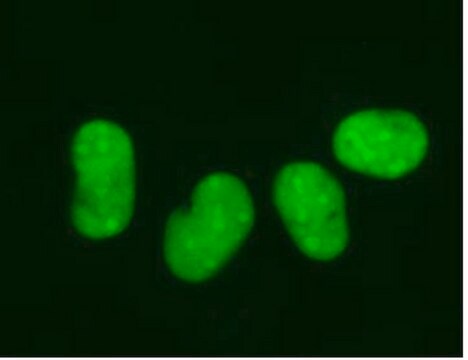
The Alzheimer’s In A Dish<TMSYMBOL></TMSYMBOL> APPSL-GFP Lentivirus is a necessary reagent to set up the Alzheimer’s 3D culture system. The lentivirus contains the APPSL FAD mutations and can be used to infect human neural cells. The TagGFP2 tag enables assessment of viral infectivity and allows FACS sorting of the highest expressing cells.
Application
Stem Cell Research
Neuroscience
Composants
Note: For exact viral titer, refer to the label on the vial.
Qualité
Stockage et stabilité
Lentivirus is stable for at least 6 months when stored at -80°C. After first thaw, place immediately on ice and store in working aliquots to avoid further freeze thaws. Avoid freeze thaws as this will result in a decrease in the virus titer.
Important Safety Note:
• Replication-defective lentiviral vectors are not known to cause any diseases in humans or animals. However, lentiviruses can integrate into the host cell genome and thus pose some risk of insertional mutagenesis.
• Wear gloves when using this product. Avoid skin contact or ingestion of all reagents and chemicals used in this protocol.
• Lentivirus is a risk group 2 and should be handled under approved Biosafety Level 2 (BSL2) controls.
Informations légales
Code de la classe de stockage
12 - Non Combustible Liquids
Certificats d'analyse (COA)
Recherchez un Certificats d'analyse (COA) en saisissant le numéro de lot du produit. Les numéros de lot figurent sur l'étiquette du produit après les mots "Lot" ou "Batch".
Déjà en possession de ce produit ?
Retrouvez la documentation relative aux produits que vous avez récemment achetés dans la Bibliothèque de documents.
Protocoles
A stem cell culture protocol to generate 3D NSC models of Alzheimer’s disease using ReNcell human neural stem cell lines.
Notre équipe de scientifiques dispose d'une expérience dans tous les secteurs de la recherche, notamment en sciences de la vie, science des matériaux, synthèse chimique, chromatographie, analyse et dans de nombreux autres domaines..
Contacter notre Service technique