219373
Cathepsin G, Human Neutrophil
Cathepsin G, Human Neutrophil, CAS 107200-92-0, is a purified native cathepsin G. Acts as a potent agonist of human platelet activation leading to their aggregation.
Synonyme(s) :
Cathepsin G, Human Neutrophil
About This Item
Produits recommandés
Source biologique
human neutrophils
Niveau de qualité
Pureté
≥95% (SDS-PAGE)
Forme
lyophilized solid (Salt-free)
Activité spécifique
≥2 units/mg protein
Fabricant/nom de marque
Calbiochem®
Conditions de stockage
OK to freeze
Technique(s)
inhibition assay: suitable
Adéquation
suitable for molecular biology
Application(s)
life science and biopharma
Conditions d'expédition
ambient
Température de stockage
−20°C
Informations sur le gène
human ... CTSG(1511)
Description générale
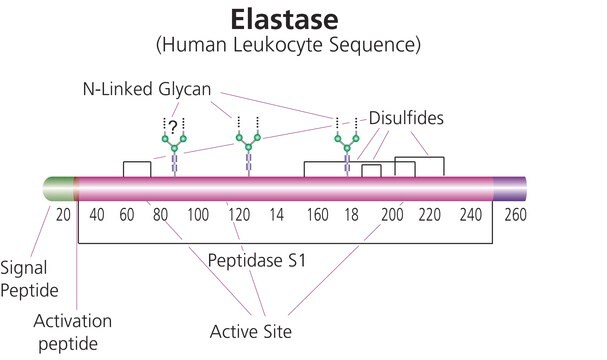
Cathepsin G is stored in its active form in azurophil granules of neutrophils or monocytes. Its mature form contains one potential glycan-binding site and three disulfide bonds.
Application
Actions biochimiques/physiologiques
Avertissement
Définition de l'unité
Notes préparatoires
Reconstitute in 150 mM NaCl, 50 mM sodium acetate buffer, pH 5.5.
Reconstitution
Autres remarques
Shamamian, P., et al. 2001. J. Cell Physiol.189, 197.
Groutas, W.C., et al. 1993. Biochem. Biophys. Res. Commun.197, 730.
Stone, P.J., et al. 1993. Biochem. Biophys. Res. Commun.197, 130.
Groutas, W.C., et al. 1992. Arch. Biochem. Biophys.294, 144.
Maison, C.M., et al. 1991. J. Immunol.147, 921.
Travis, J. 1988. Am. J. Med.84, 37.
Informations légales
Code de la classe de stockage
11 - Combustible Solids
Classe de danger pour l'eau (WGK)
WGK 3
Point d'éclair (°F)
Not applicable
Point d'éclair (°C)
Not applicable
Certificats d'analyse (COA)
Recherchez un Certificats d'analyse (COA) en saisissant le numéro de lot du produit. Les numéros de lot figurent sur l'étiquette du produit après les mots "Lot" ou "Batch".
Déjà en possession de ce produit ?
Retrouvez la documentation relative aux produits que vous avez récemment achetés dans la Bibliothèque de documents.
Notre équipe de scientifiques dispose d'une expérience dans tous les secteurs de la recherche, notamment en sciences de la vie, science des matériaux, synthèse chimique, chromatographie, analyse et dans de nombreux autres domaines..
Contacter notre Service technique