1.07531
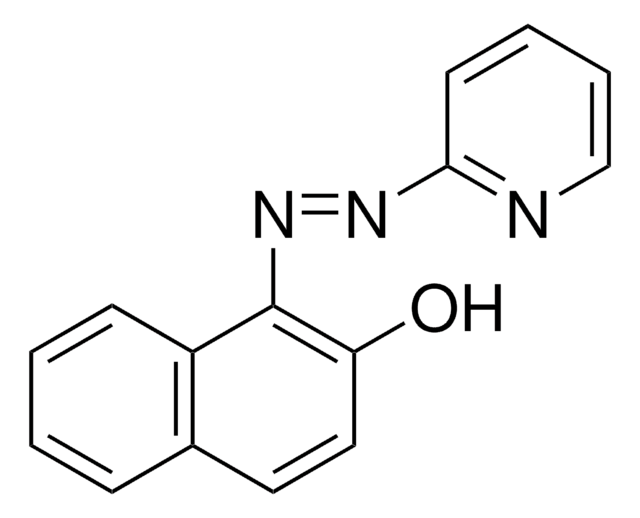
1-(2-Pyridylazo)-2-naphthol
(PAN) metal indicator, Reag. Ph Eur
Synonyme(s) :
1-(2-Pyridylazo)-2-naphthol (PAN), PAN
About This Item
Produits recommandés
Nom du produit
1-(2-Pyridylazo)-2-naphthol (PAN), metal indicator Reag. Ph Eur
Agence
reag. Ph. Eur.
Niveau de qualité
Forme
solid
Couleur
brick red to orange-brown
clear to almost clear red-orange to brown-orange, 1.0g/L;ethanol
Pf
136-141 °C
Masse volumique apparente
190 kg/m3
λmax
461-465 nm in ethanol
Température de stockage
2-30°C
InChI
1S/C15H11N3O/c19-13-9-8-11-5-1-2-6-12(11)15(13)18-17-14-7-3-4-10-16-14/h1-10H,(H,16,17)/b18-15-
Clé InChI
RAXUMGMWXZYADR-SDXDJHTJSA-N
Vous recherchez des produits similaires ? Visite Guide de comparaison des produits
Catégories apparentées
Application
- Derivative spectrophotometric determination of iridium after preconcentration of its 1-(2-pyridylazo)-2-naphthol complex on microcrystalline naphthalene.: This study explores a spectrophotometric method for determining iridium using 1-(2-Pyridylazo)-2-naphthol (PAN) as a complexing agent, demonstrating an effective approach for trace metal analysis in academic and research applications, highlighting its relevance to environmental monitoring and analytical chemistry (Taher MA et al., 1997).
- Simultaneous determination of gallium(III) and indium(III) by derivative spectrophotometry.: This research presents a derivative spectrophotometric technique for the concurrent determination of gallium and indium, employing PAN for chelation, underscoring its utility in the precise analysis of these metals in various sample matrices, which is crucial for chemists working in analytical fields (Singh VK et al., 2001).
- Second and first-derivative spectrophotometry for efficient simultaneous and individual determination of palladium and cobalt using 1-(2-pyridylazo)-2-naphthol in sodium dodecylsulfate micellar media.: This paper introduces a novel approach for the simultaneous spectrophotometric determination of palladium and cobalt using PAN, offering significant advantages in terms of sensitivity and selectivity, essential for advancing analytical methodologies in chemistry (Eskandari H et al., 2003).
- Zero-crossing second derivative spectrophotometry for simultaneous determination of palladium and nickel using 1-(2-pyridylazo)-2-naphthol in mixed micellar solutions.: This publication details a method for analyzing palladium and nickel using PAN, employing advanced spectrophotometric techniques to enhance detection capabilities, reflecting critical developments in analytical techniques relevant to chemists in academic settings (Eskandari H, 2004).
- Preconcentration of 1-(2-pyridylazo)-2-naphthol-iron(III)-capriquat on a membrane filter, and third-derivative spectrophotometric determination of iron(III).: The study focuses on a preconcentration technique for iron analysis using PAN, demonstrating the application of derivative spectrophotometry in enhancing analytical precision, an essential aspect for researchers focused on trace metal analysis in environmental and industrial samples (Mori I et al., 1994).
Remarque sur l'analyse
Appearance: brick-red to orange-brown fine powder,eventually cohesive
Appearance of solution (1.0 g/l; ethanol): Clear to almost clear, red-orange to brown-orange
Melting point: 136 - 141 °C
Absorption maximum λmax. (Ethanol): 461 - 465 nm
Spec. Absorptivity A 1%/1cm (λmax; 0.01 g/l; Ethanol): 670 - 720
Suitability as indicator (for metal titration): passes test
Sensitivity test (Reag. Ph Eur): passes test
Code de la classe de stockage
11 - Combustible Solids
Classe de danger pour l'eau (WGK)
WGK 3
Point d'éclair (°F)
Not applicable
Point d'éclair (°C)
Not applicable
Certificats d'analyse (COA)
Recherchez un Certificats d'analyse (COA) en saisissant le numéro de lot du produit. Les numéros de lot figurent sur l'étiquette du produit après les mots "Lot" ou "Batch".
Déjà en possession de ce produit ?
Retrouvez la documentation relative aux produits que vous avez récemment achetés dans la Bibliothèque de documents.
Notre équipe de scientifiques dispose d'une expérience dans tous les secteurs de la recherche, notamment en sciences de la vie, science des matériaux, synthèse chimique, chromatographie, analyse et dans de nombreux autres domaines..
Contacter notre Service technique