P-063
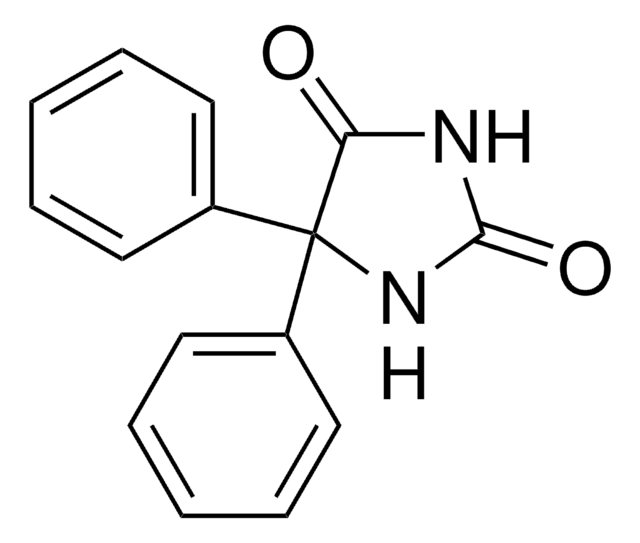
Phenytoin solution
1.0 mg/mL in methanol, ampule of 1 mL, certified reference material, Cerilliant®
About This Item
Produits recommandés
Qualité
certified reference material
Niveau de qualité
Forme
liquid
Caractéristiques
Snap-N-Spike®/Snap-N-Shoot®
Conditionnement
ampule of 1 mL
Fabricant/nom de marque
Cerilliant®
Concentration
1.0 mg/mL in methanol
Technique(s)
gas chromatography (GC): suitable
liquid chromatography (LC): suitable
Application(s)
clinical testing
Format
single component solution
Température de stockage
−20°C
Chaîne SMILES
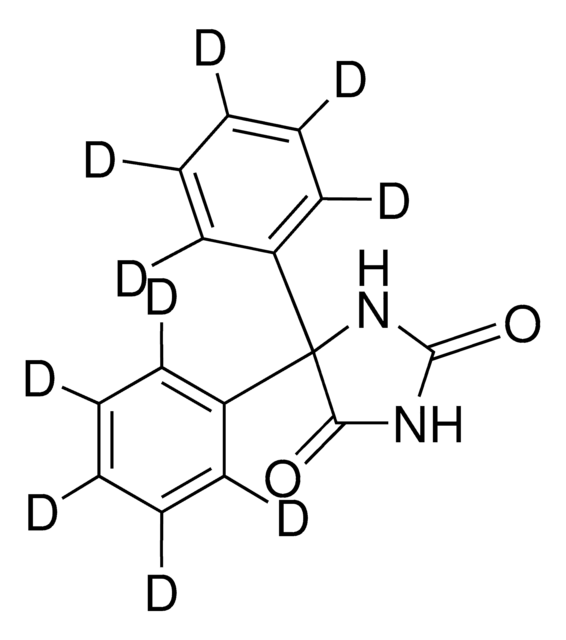
O=C1NC(=O)C(N1)(c2ccccc2)c3ccccc3
InChI
1S/C15H12N2O2/c18-13-15(17-14(19)16-13,11-7-3-1-4-8-11)12-9-5-2-6-10-12/h1-10H,(H2,16,17,18,19)
Clé InChI
CXOFVDLJLONNDW-UHFFFAOYSA-N
Informations sur le gène
human ... SCN10A(6336) , SCN11A(11280) , SCN1A(6323) , SCN2A(6326) , SCN3A(6328) , SCN4A(6329) , SCN5A(6331) , SCN7A(6332) , SCN8A(6334) , SCN9A(6335)
Description générale
Application
- Advanced Separation and Extraction of Anticonvulsants: Phenytoin solution is utilized in the fabrication of surface molecularly imprinted polymer membranes. This innovative method enhances the separation and extraction of phenytoin, phenobarbital, and lamotrigine, significantly improving the precision and efficiency of drug monitoring in clinical and research settings (Zhao et al., 2024).
- LC-MS/MS Quantification for Drug Delivery: A sensitive LC-MS/MS method has been developed for the quantification of phenytoin and its major metabolite. This method is applied to both intravenous and intranasal delivery systems, enhancing our understanding of the pharmacokinetics and effectiveness of phenytoin as an anticonvulsant medication (Prentice et al., 2022).
- Clinical Decision Support for Pharmacogenomics: Phenytoin is involved in the appraisal and development of evidence-based clinical decision support systems. This research integrates pharmacogenomic data to optimize perioperative drug use, providing tailored therapeutic strategies based on genetic profiles, particularly for managing epilepsy treatments (Borden et al., 2021).
- Electromembrane Extraction Technique: The development of an electromembrane extraction method followed by capillary electrophoresis for phenytoin determination in exhaled breath condensate. This technique offers a non-invasive approach for therapeutic drug monitoring, critical for ensuring effective seizure control in epilepsy patients (Seyfinejad et al., 2020).
- Drug Crystallization Inhibition: Research into the use of polymer nanogels as reservoirs for phenytoin has shown potential in inhibiting drug crystallization. This approach could revolutionize drug delivery systems by maintaining the stability and bioavailability of phenytoin, enhancing its therapeutic efficacy for treating neurological disorders (Li et al., 2019).
Informations légales
Produit(s) apparenté(s)
Mention d'avertissement
Danger
Mentions de danger
Conseils de prudence
Classification des risques
Acute Tox. 3 Dermal - Acute Tox. 3 Inhalation - Acute Tox. 3 Oral - Flam. Liq. 2 - STOT SE 1
Organes cibles
Eyes,Central nervous system
Code de la classe de stockage
3 - Flammable liquids
Classe de danger pour l'eau (WGK)
WGK 2
Point d'éclair (°F)
49.5 °F - closed cup
Point d'éclair (°C)
9.7 °C - closed cup
Certificats d'analyse (COA)
Recherchez un Certificats d'analyse (COA) en saisissant le numéro de lot du produit. Les numéros de lot figurent sur l'étiquette du produit après les mots "Lot" ou "Batch".
Déjà en possession de ce produit ?
Retrouvez la documentation relative aux produits que vous avez récemment achetés dans la Bibliothèque de documents.
Les clients ont également consulté
Notre équipe de scientifiques dispose d'une expérience dans tous les secteurs de la recherche, notamment en sciences de la vie, science des matériaux, synthèse chimique, chromatographie, analyse et dans de nombreux autres domaines..
Contacter notre Service technique