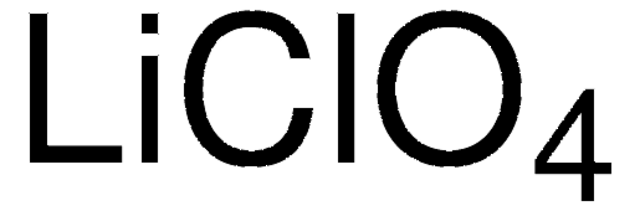931969
Lithium perchlorate
anhydrous, ≥99.9% trace metals basis
Synonyme(s) :
Perchloric acid lithium salt
About This Item
Produits recommandés
Qualité
anhydrous
battery grade
Niveau de qualité
Pureté
≥99.9% trace metals basis
Forme
powder
Caractéristiques du produit alternatif plus écologique
Design for Energy Efficiency
Learn more about the Principles of Green Chemistry.
sustainability
Greener Alternative Product
Impuretés
≤1000 ppm (trace metals analysis)
pH
6.0-7.5 (25 °C, 5%, aq.sol.)
Pf
236 °C (lit.)
Solubilité
H2O: 59.8 g/dL at 25 °C
Traces d'anions
chloride (Cl-): ≤30 ppm
sulfate (SO42-): ≤10 ppm
Traces de cations
Fe: ≤5 ppm
heavy metals: ≤10 ppm
Application(s)
battery manufacturing
Autre catégorie plus écologique
Chaîne SMILES
[Li+].[O-]Cl(=O)(=O)=O
InChI
1S/ClHO4.Li/c2-1(3,4)5;/h(H,2,3,4,5);/q;+1/p-1
Clé InChI
MHCFAGZWMAWTNR-UHFFFAOYSA-M
Vous recherchez des produits similaires ? Visite Guide de comparaison des produits
Description générale
Industrially, lithium perchlorate is manufactured in several ways. Most commonly, it is prepared from sodium perchlorate through a metathesis reaction with lithium chloride or lithium carbonate. Lithium perchlorate can also be prepared by direct electrochemical oxidation of lithium chloride or by reacting lithium carbonate with perchloric acid. The hydrate can be dried either by highly controlled heating or by displacing water with volatile amines, which are removed by drying under vacuum.
Application
Researchers also use lithium perchlorate as an electrolytic salt in aqueous media when testing electrocatalysts. For example, recent experiments improving the electrochemical reduction of nitrogen over TiO2 nanoparticles or gold nanoparticles use aqueous lithium perchlorate as the electrolyte.
Conditionnement
500g in poly bottle
Mention d'avertissement
Danger
Mentions de danger
Conseils de prudence
Classification des risques
Acute Tox. 4 Oral - Eye Dam. 1 - Ox. Sol. 2 - Skin Corr. 1A - STOT SE 3
Organes cibles
Respiratory system
Code de la classe de stockage
5.1A - Strongly oxidizing hazardous materials
Classe de danger pour l'eau (WGK)
WGK 1
Point d'éclair (°F)
Not applicable
Point d'éclair (°C)
Not applicable
Faites votre choix parmi les versions les plus récentes :
Certificats d'analyse (COA)
Désolés, nous n'avons pas de COA pour ce produit disponible en ligne pour le moment.
Si vous avez besoin d'assistance, veuillez contacter Service Clients
Déjà en possession de ce produit ?
Retrouvez la documentation relative aux produits que vous avez récemment achetés dans la Bibliothèque de documents.
Notre équipe de scientifiques dispose d'une expérience dans tous les secteurs de la recherche, notamment en sciences de la vie, science des matériaux, synthèse chimique, chromatographie, analyse et dans de nombreux autres domaines..
Contacter notre Service technique