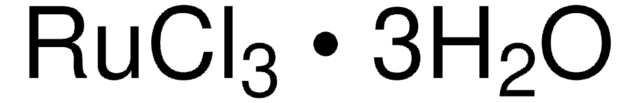931578
Ruthenium(III) chloride hydrate
≥99.9% trace metals basis
Synonyme(s) :
Ruthenium trichloride, Trichlororuthenium hydrate
About This Item
Produits recommandés
Qualité
for analytical purposes
Niveau de qualité
Essai
≥99.9% trace metals basis
Forme
powder
Impuretés
≤1000.0 ppm (trace metals analysis)
Solubilité
acetone: soluble ((lit.))
ethanol: soluble
water: soluble
Densité
3.11 g/cm3
Chaîne SMILES
O.Cl[Ru](Cl)Cl
InChI
1S/3ClH.H2O.Ru/h3*1H;1H2;/q;;;;+3/p-3
Clé InChI
BIXNGBXQRRXPLM-UHFFFAOYSA-K
Vous recherchez des produits similaires ? Visite Guide de comparaison des produits
Catégories apparentées
Description générale
Industrially, ruthenium trichloride hydrate is produced by dissolving ruthenium oxides in hydrochloric acid. The hydrated salt is obtained by recrystallization.
Application
Another common application of ruthenium chloride hydrate is as a precursor for single-atom catalysts. For example, scientists have used ruthenium chloride hydrate for the synthesis of ruthenium single-atom-doped ZrO2 particles to catalyze nitrogen fixation and for the synthesis of ruthenium single-atom-doped MXenes to catalyze hydrogen evolution. A third common application of ruthenium chloride hydrate is in the synthesis of metal alloys, like PtRuIr, or PtRuFe, which are investigated for electrocatalysis, usually the oxidation of simple organics like methanol or formic acid.
For use in all these applications, also consider our higher-purity ruthenium chloride hydrate, 463779, with trace metals purity greater than 99.98%, which offers the best reproducibility and purity.
Mention d'avertissement
Danger
Mentions de danger
Conseils de prudence
Classification des risques
Acute Tox. 4 Oral - Aquatic Chronic 2 - Eye Dam. 1 - Skin Corr. 1B
Code de la classe de stockage
8A - Combustible corrosive hazardous materials
Classe de danger pour l'eau (WGK)
WGK 3
Point d'éclair (°F)
Not applicable
Point d'éclair (°C)
Not applicable
Faites votre choix parmi les versions les plus récentes :
Certificats d'analyse (COA)
It looks like we've run into a problem, but you can still download Certificates of Analysis from our Documents section.
Si vous avez besoin d'assistance, veuillez contacter Service Clients
Déjà en possession de ce produit ?
Retrouvez la documentation relative aux produits que vous avez récemment achetés dans la Bibliothèque de documents.
Notre équipe de scientifiques dispose d'une expérience dans tous les secteurs de la recherche, notamment en sciences de la vie, science des matériaux, synthèse chimique, chromatographie, analyse et dans de nombreux autres domaines..
Contacter notre Service technique