791989
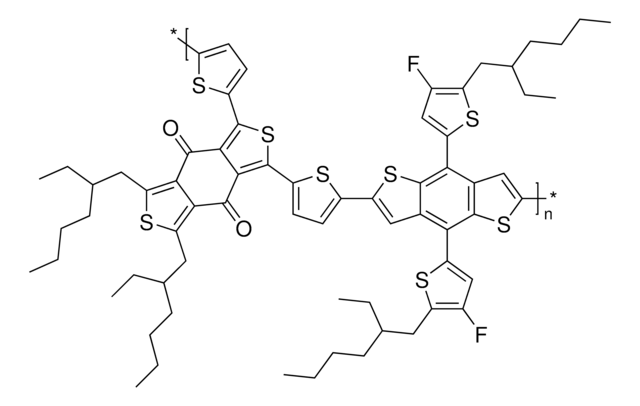
PDPP2T-TT-OD
Synonyme(s) :
Diketopyrrolopyrrole - thienothiophene copolymer, Diketopyrrolopyrrole p-type semiconductor, Xerox XSC4p
About This Item
Produits recommandés
Forme
solid
Poids mol.
average Mw 40,000-60,000 by GPC
Couleur
dark blue
Pf
>200 °C
λmax
820 nm (thin film)
Énergie orbitale
HOMO 5.2 eV
Performance des dispositifs OFET
Bottom gate top contact device with silane modified SiO2 dielectric.Processing method spin coating; thermal annealing at 140 °C/ 10 min
PDI
2.5‑3.0
Description générale
Application
monolayer. An XSC4p film was deposited by spin-coating a 0.5 – 0.7 wt. % polymer solution at 1000 rpm for 60 sec. The film was dried in a vacuum oven for 10 min at 80
°C and then thermally annealed at 140 °C for 10 min. Gold source-drain electrodes were deposited by vacuum evaporation using a shadow mask (90 micrometer channels).
The electrical performance was characterized using a Keithley SCS-4200 system. The reported mobility is the average saturated mobility of four devicεs ± the standard
deviation.
Informations légales
Mention d'avertissement
Warning
Mentions de danger
Conseils de prudence
Classification des risques
Eye Irrit. 2 - Skin Irrit. 2 - STOT SE 3
Organes cibles
Respiratory system
Code de la classe de stockage
11 - Combustible Solids
Classe de danger pour l'eau (WGK)
WGK 3
Point d'éclair (°F)
Not applicable
Point d'éclair (°C)
Not applicable
Faites votre choix parmi les versions les plus récentes :
Déjà en possession de ce produit ?
Retrouvez la documentation relative aux produits que vous avez récemment achetés dans la Bibliothèque de documents.
Articles
The development of high-performance conjugated organic molecules and polymers has received widespread attention in industrial and academic research.
Notre équipe de scientifiques dispose d'une expérience dans tous les secteurs de la recherche, notamment en sciences de la vie, science des matériaux, synthèse chimique, chromatographie, analyse et dans de nombreux autres domaines..
Contacter notre Service technique



![[6,6]-Phenyl C71 butyric acid methyl ester 99%](/deepweb/assets/sigmaaldrich/product/structures/716/624/9fb9f2f0-ae99-429f-8d3a-b12267976a4d/640/9fb9f2f0-ae99-429f-8d3a-b12267976a4d.png)