670359
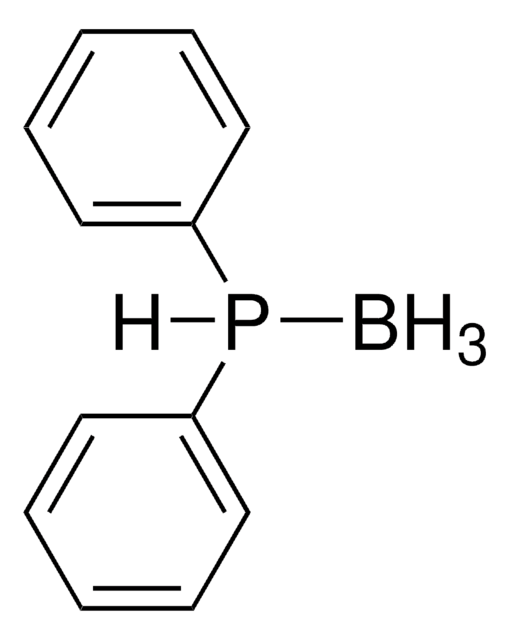
Acetylthiomethyl-diphenylphosphine borane complex
≥98.0%
Synonyme(s) :
(T-4)-[S-[(Diphenylphosphino-κP)methyl] ethanethioate]trihydroboron
About This Item
Produits recommandés
Pureté
≥98.0%
Forme
solid
Capacité de réaction
reaction type: click chemistry
Pertinence de la réaction
reagent type: ligand
reaction type: Staudinger Reaction
Pf
52-55 °C
Groupe fonctionnel
phosphine
Température de stockage
2-8°C
Chaîne SMILES
B.CC(=O)SCP(c1ccccc1)c2ccccc2
InChI
1S/C15H15OPS.BH3/c1-13(16)18-12-17(14-8-4-2-5-9-14)15-10-6-3-7-11-15;/h2-11H,12H2,1H3;1H3
Clé InChI
MXPNVFCCEGQGEN-UHFFFAOYSA-N
Application
- Traceless Staudinger ligation reagent with borane protecting group.
- The borane group stabilizes the phosphine against oxidation and can be easily removed with mild basic or acidic conditions to yield the active phosphine.
- After reaction with an azide, the phosphine is eliminated in the presence of water to yield a native amide bond.
- Used in the synthesis of cyclic peptides.

Conditionnement
Informations légales
Produit(s) apparenté(s)
Mention d'avertissement
Warning
Mentions de danger
Conseils de prudence
Classification des risques
Eye Irrit. 2 - Skin Irrit. 2 - STOT SE 3
Organes cibles
Respiratory system
Code de la classe de stockage
11 - Combustible Solids
Classe de danger pour l'eau (WGK)
WGK 3
Point d'éclair (°F)
Not applicable
Point d'éclair (°C)
Not applicable
Équipement de protection individuelle
dust mask type N95 (US), Eyeshields, Gloves
Certificats d'analyse (COA)
Recherchez un Certificats d'analyse (COA) en saisissant le numéro de lot du produit. Les numéros de lot figurent sur l'étiquette du produit après les mots "Lot" ou "Batch".
Déjà en possession de ce produit ?
Retrouvez la documentation relative aux produits que vous avez récemment achetés dans la Bibliothèque de documents.
Articles
Traceless Staudinger Ligation
Based on the same working principle as the nontraceless Staudinger Ligation the auxiliary phosphine reagent can be cleaved from the product after the ligation is completed leaving a native amide bond. Thus, the total chemical synthesis of proteins and glycopeptides is enabled overcoming the limitations of native chemical ligation (NCL) of a Cys residue at the ligation juncture.
Chemoselective ligation strategies are a key success factor for chemical biology research. Ligation techniques open pathways to fully synthetic large peptides and even proteins.
The reaction between an azide and a phosphine forming an aza-ylide was discovered almost a century ago by Nobel Prize laureate Herrmann Staudinger.
Notre équipe de scientifiques dispose d'une expérience dans tous les secteurs de la recherche, notamment en sciences de la vie, science des matériaux, synthèse chimique, chromatographie, analyse et dans de nombreux autres domaines..
Contacter notre Service technique