441236
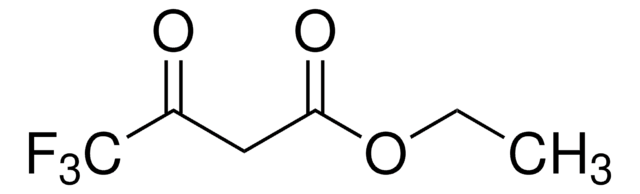
Ethyl 2-(ethoxymethylene)-4,4,4-trifluoro-3-oxobutyrate,mixture of cis and trans
96%
Se connecterpour consulter vos tarifs contractuels et ceux de votre entreprise/organisme
About This Item
Formule linéaire :
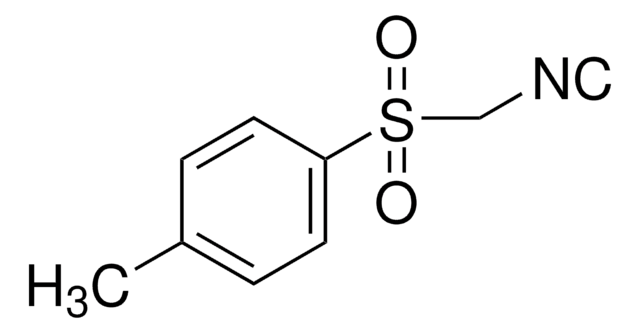
CF3COC(=CHOC2H5)CO2C2H5
Numéro CAS:
Poids moléculaire :
240.18
Numéro MDL:
Code UNSPSC :
12352100
ID de substance PubChem :
Nomenclature NACRES :
NA.22
Produits recommandés
Niveau de qualité
Pureté
96%
Forme
liquid
Indice de réfraction
n20/D 1.429 (lit.)
Point d'ébullition
80-82 °C/1 mmHg (lit.)
Densité
1.235 g/mL at 25 °C (lit.)
Température de stockage
2-8°C
Chaîne SMILES
CCO\C=C(\C(=O)OCC)C(=O)C(F)(F)F
InChI
1S/C9H11F3O4/c1-3-15-5-6(8(14)16-4-2)7(13)9(10,11)12/h5H,3-4H2,1-2H3/b6-5+
Clé InChI
XNGGOXOLHQANRB-AATRIKPKSA-N
Description générale
Ethyl 2-(ethoxymethylene)-4,4,4-trifluoro-3-oxobutyrate has been reported to participate in the microwave-assisted synthesis of ethyl 1-[4-(2,3,3-trichloroacrylamido)phenyl]-5-(trifluoromethyl)-1H-pyrazole-4-carboxylate.
Application
Ethyl 2-(ethoxymethylene)-4,4,4-trifluoro-3-oxobutyrate may be employed as a starting reagent for the synthesis of 1-methyl-3-trifluoromethyl-1H-pyrazole-4- carboxylic acid.
Code de la classe de stockage
10 - Combustible liquids
Classe de danger pour l'eau (WGK)
WGK 3
Point d'éclair (°F)
219.2 °F - closed cup
Point d'éclair (°C)
104.00 °C - closed cup
Équipement de protection individuelle
Eyeshields, Gloves
Faites votre choix parmi les versions les plus récentes :
Déjà en possession de ce produit ?
Retrouvez la documentation relative aux produits que vous avez récemment achetés dans la Bibliothèque de documents.
L Sansebastiano et al.
Farmaco (Societa chimica italiana : 1989), 48(3), 335-355 (1993-03-01)
The synthesis of ethyl or methyl 4-substituted or unsubstituted 2-methylthio-5-pyrimidinecarboxylates 3 a-i and 8 o mainly by reaction of ethyl or methyl 2-dimethylaminomethylene-3-oxoalkanoates with 2-methylisothiourea is described. Also some ethyl 2-substituted (NH2, CH3, C6H5) 4-trifluoromethyl-5-pyrimidinecarboxylates were prepared. Some of the
P J Sanfilippo et al.
Journal of medicinal chemistry, 38(1), 34-41 (1995-01-06)
The synthesis and biological activity of novel thiazole-based heterocycles as inhibitors of thrombin-induced human platelet aggregation are described. Further evaluation of selected compounds show they inhibit platelet aggregation as stimulated by a variety of agonists. The more active compounds also
R D Franz
AAPS pharmSci, 3(2), E10-E10 (2001-12-14)
The changes in the physiochemical properties accompanying the substitution of a phosphonic acid group for a carboxylic acid group on various heterocyclic platforms was determined. A series of low molecular weight heterocyclic carboxylic and phosphonic acids was prepared, and the
L Mosti et al.
Farmaco (Societa chimica italiana : 1989), 47(4), 427-437 (1992-04-01)
The synthesis of ethyl or methyl esters of 5-cyano-1,6-dihydro-6-oxo-3- pyridinecarboxylic acids carrying as 2-substituent a polar group such as CO2C2H5, (CH2)2CO2CH3, (CH2)3CO2C2H5, CH2OCH3, or CF3 group is described. Also 2-[5-cyano-1,6-dihydro-2-(1,1-dimethylethyl)-6-oxo-3-pyridyl]-2- oxoacetic acid and 2,5,6,8-tetrahydro-2,5-dioxo-1H-thiopyrano[3,4-b]pyridine-3-carbon itrile were prepared. Nearly all the
European Journal of Medicinal Chemistry, 28, 853-853 (1993)
Notre équipe de scientifiques dispose d'une expérience dans tous les secteurs de la recherche, notamment en sciences de la vie, science des matériaux, synthèse chimique, chromatographie, analyse et dans de nombreux autres domaines..
Contacter notre Service technique