433101
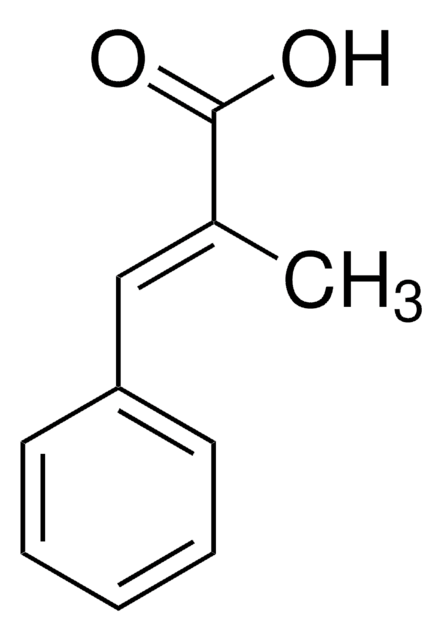
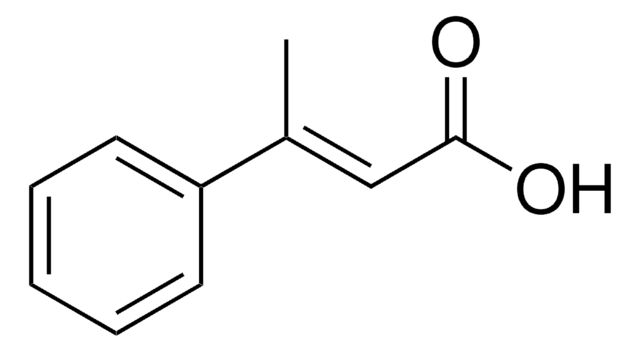
2-Methylcinnamic acid, predominantly trans
99%
Se connecterpour consulter vos tarifs contractuels et ceux de votre entreprise/organisme
About This Item
Formule linéaire :
CH3C6H4CH=CHCO2H
Numéro CAS:
Poids moléculaire :
162.19
Numéro CE :
Numéro MDL:
Code UNSPSC :
12352100
ID de substance PubChem :
Nomenclature NACRES :
NA.22
Produits recommandés
Niveau de qualité
Pureté
99%
Pf
174-176 °C (lit.)
Groupe fonctionnel
carboxylic acid
Chaîne SMILES
Cc1ccccc1\C=C\C(O)=O
InChI
1S/C10H10O2/c1-8-4-2-3-5-9(8)6-7-10(11)12/h2-7H,1H3,(H,11,12)/b7-6+
Clé InChI
RSWBWHPZXKLUEX-VOTSOKGWSA-N
Description générale
2-Methylcinnamic acid has been reported to exhibit strong anti-fungal activity against white-rot fungus Lenzites betulina and brown-rot fungus Laetiporus sulphureus. Hydrogenation of 2-methylcinnamic acid using Walphos ligands and their biferrocene analogs has been studied.
Application
2-Methylcinnamic acid may be used as starting reagent for the total synthesis of the cytotoxic alkaloid, 22-hydroxyacuminatine and for the preparation of (E)-2-methylcinnamic acid i-butylammonium salt.
Code de la classe de stockage
11 - Combustible Solids
Classe de danger pour l'eau (WGK)
WGK 3
Point d'éclair (°F)
Not applicable
Point d'éclair (°C)
Not applicable
Équipement de protection individuelle
Eyeshields, Gloves, type N95 (US)
Faites votre choix parmi les versions les plus récentes :
Déjà en possession de ce produit ?
Retrouvez la documentation relative aux produits que vous avez récemment achetés dans la Bibliothèque de documents.
Xiangshu Xiao et al.
Journal of medicinal chemistry, 49(4), 1408-1412 (2006-02-17)
A total synthesis of 22-hydroxyacuminatine, a cytotoxic alkaloid isolated from Camptotheca acuminata, is reported. The key step in the synthesis involves the reaction of 2,3-dihydro-1H-pyrrolo[3,4-b]quinoline with a brominated phthalide to generate a substituted pentacyclic 12H-5,11a-diazadibenzo[b,h]fluoren-11-one intermediate. Despite its structural resemblance
Sen-Sung Cheng et al.
Bioresource technology, 99(11), 5145-5149 (2007-10-20)
In this study, the antifungal activities of cinnamaldehyde and eugenol congeners against white-rot fungus Lenzites betulina and brown-rot fungus Laetiporus sulphureus were evaluated and the relationships between the antifungal activity and the chemical structures were also examined. Results from antifungal
Martin E Fox et al.
The Journal of organic chemistry, 73(3), 775-784 (2007-10-24)
Four chiral diphosphine ligands consisting of bis(2,5-diphenylphospholan-1-yl) groups connected by the sp(2) carbon linkers 2,3-quinoxaline ((S,S)-Ph-Quinox), 2,3-pyrazine ((S,S)-Ph-Pyrazine), maleic anhydride ((S,S)-Ph-MalPhos), and 1,1'-ferrocene ((S,S)-Ph-5-Fc) were synthesized, and their cationic [rhodium(I)(COD)] complexes were prepared. These complexes were tested in asymmetric hydrogenation
Afrooz Zirakzadeh et al.
Organometallics, 33(8), 1945-1952 (2014-05-06)
Two representative Walphos analogues with an achiral 2,2″-biferrocenediyl backbone were synthesized. These diphosphine ligands were tested in the rhodium-catalyzed asymmetric hydrogenation of several alkenes and in the ruthenium-catalyzed hydrogenation of two ketones. The results were compared with those previously obtained
Notre équipe de scientifiques dispose d'une expérience dans tous les secteurs de la recherche, notamment en sciences de la vie, science des matériaux, synthèse chimique, chromatographie, analyse et dans de nombreux autres domaines..
Contacter notre Service technique