374776
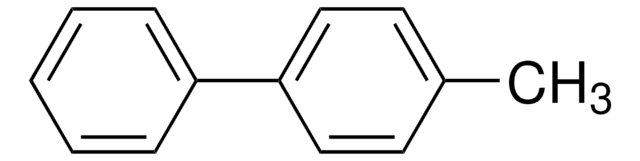
4-Methoxybiphenyl
97%
Synonyme(s) :
4-Phenylanisole
Se connecterpour consulter vos tarifs contractuels et ceux de votre entreprise/organisme
About This Item
Formule linéaire :
C6H5C6H4OCH3
Numéro CAS:
Poids moléculaire :
184.23
Numéro CE :
Numéro MDL:
Code UNSPSC :
12171500
ID de substance PubChem :
Nomenclature NACRES :
NA.47
Produits recommandés
Pureté
97%
Forme
powder, crystals or chunks
Pf
86-90 °C (lit.)
Application(s)
diagnostic assay manufacturing
hematology
histology
Température de stockage
room temp
Chaîne SMILES
COc1ccc(cc1)-c2ccccc2
InChI
1S/C13H12O/c1-14-13-9-7-12(8-10-13)11-5-3-2-4-6-11/h2-10H,1H3
Clé InChI
RHDYQUZYHZWTCI-UHFFFAOYSA-N
Application
4-Methoxybiphenyl has been used as a standard reagent whose fluorescence intensity is associated with the fluorescence characteristics of the products of derivatization reaction for aryl halides with phenylboronic acid (PBA).
Code de la classe de stockage
11 - Combustible Solids
Classe de danger pour l'eau (WGK)
WGK 3
Point d'éclair (°F)
Not applicable
Point d'éclair (°C)
Not applicable
Équipement de protection individuelle
Eyeshields, Gloves, type N95 (US)
Faites votre choix parmi les versions les plus récentes :
Déjà en possession de ce produit ?
Retrouvez la documentation relative aux produits que vous avez récemment achetés dans la Bibliothèque de documents.
Les clients ont également consulté
J R Fry
Xenobiotica; the fate of foreign compounds in biological systems, 17(6), 751-758 (1987-06-01)
1. The metabolism of 4-methoxybiphenyl to 4-hydroxybiphenyl and its sulphate and glucuronic acid conjugates has been studied in rat isolated hepatocytes at various concentrations of 4-methoxybiphenyl. 2. The proportions of metabolites produced remained constant at concentrations of 4-methoxybiphenyl less than
A comparison of biphenyl 4-hydroxylation and 4-methoxybiphenyl O-demethylation in rat liver microsomes.
J R Fry
Biochemical pharmacology, 30(14), 1915-1919 (1981-07-15)
P Paterson et al.
Xenobiotica; the fate of foreign compounds in biological systems, 15(6), 493-502 (1985-06-01)
The rate of production of 4-hydroxybiphenyl from 4-methoxybiphenyl in hepatocytes isolated from untreated rats was essentially identical to that from biphenyl in hepatocytes isolated from rats pretreated with beta-naphthoflavone at 40 mg/kg. Similar results were obtained using liver microsomes isolated
Influence of the sulphation inhibitor, 2,6-dichloro-4-nitrophenol, on the production and conjugation, of 4-hydroxybiphenyl generated from 4-methoxybiphenyl by rat isolated hepatocytes.
J R Fry et al.
Biochemical pharmacology, 36(18), 3090-3092 (1987-09-15)
Notre équipe de scientifiques dispose d'une expérience dans tous les secteurs de la recherche, notamment en sciences de la vie, science des matériaux, synthèse chimique, chromatographie, analyse et dans de nombreux autres domaines..
Contacter notre Service technique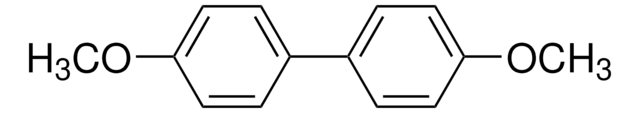


![1-(4′-Methyl[1,1′-biphenyl]-4-yl)ethanone AldrichCPR](/deepweb/assets/sigmaaldrich/product/structures/224/118/064dfb31-1067-44ce-bf18-d3f762028eb6/640/064dfb31-1067-44ce-bf18-d3f762028eb6.png)