318183
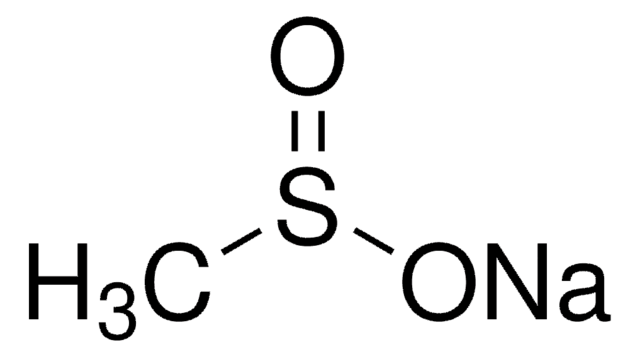
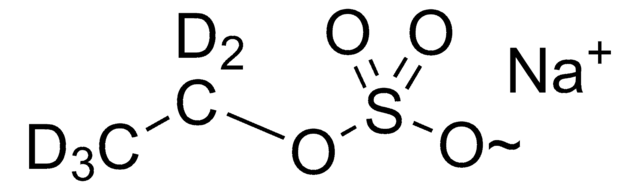
Methyl sulfate sodium salt
Synonyme(s) :
Monomethyl ester sulfuric acid sodium salt, Sodium methyl sulfate
About This Item
Produits recommandés
Forme
powder
Impuretés
<3% methanol
<5% water
Pf
210 °C (lit.)
Chaîne SMILES
[Na+].COS([O-])(=O)=O
InChI
1S/CH4O4S.Na/c1-5-6(2,3)4;/h1H3,(H,2,3,4);/q;+1/p-1
Clé InChI
DZXBHDRHRFLQCJ-UHFFFAOYSA-M
Application
- Methylsulfate anion based 1,3,4-trialkyl-1,2,3-triazolium ionic liquids for use in Morita–Baylis–Hillman reaction.
- Anisole by reacting with phenol.
- Hydroxyanilino quinolines for use as RET kinase inhibitors.
- Studying lamellar structure formation in hybrid nanomaterials created by miniemulsion
- EPR studies of radical ions radiolytically generated from ionic liquids, used as a reference
- Micellular studies specifically the rate-retarding effects on hydrolysis of substituted 1-benzoyl-1,2,4-triazoles and self-assembly / microstructure of mixed micelles
Mention d'avertissement
Warning
Mentions de danger
Conseils de prudence
Classification des risques
Acute Tox. 4 Oral - Skin Irrit. 2 - STOT SE 3
Organes cibles
Respiratory system
Code de la classe de stockage
11 - Combustible Solids
Classe de danger pour l'eau (WGK)
WGK 3
Point d'éclair (°F)
Not applicable
Point d'éclair (°C)
Not applicable
Équipement de protection individuelle
dust mask type N95 (US), Eyeshields, Gloves
Faites votre choix parmi les versions les plus récentes :
Déjà en possession de ce produit ?
Retrouvez la documentation relative aux produits que vous avez récemment achetés dans la Bibliothèque de documents.
Les clients ont également consulté
Notre équipe de scientifiques dispose d'une expérience dans tous les secteurs de la recherche, notamment en sciences de la vie, science des matériaux, synthèse chimique, chromatographie, analyse et dans de nombreux autres domaines..
Contacter notre Service technique